CHAPTER 13 Induction, Maintenance, and Recovery
Induction of general anesthesia
The induction of general anesthesia can be the most critical yet rewarding interaction that a pediatric anesthesiologist has with young patients and their families. Minimizing anxiety, psychological trauma, and crying during induction has many advantages, including reduced occurrence of airway complications, emergence agitation, postoperative pain, short- and long-term behavioral changes, patient and family dissatisfaction, and difficult inductions with subsequent anesthetics (Laycock and McNicol, 1988; Kotiniemi et al., 1997; Kain et al., 1999; Przybylo et al., 2005; Kain et al., 2006). With the increased focus on family-centered care, including parents’ concerns and priorities in the decision-making process being the norm, it is essential that all parties are prepared as well as possible regarding information, preferences, and expectations. If premedications or topical anesthetics are indicated, adequate time should be allotted to ensure maximal benefit. Smooth separation of child and family, be it before or after induction, should be facilitated by clearly stated instructions and participation of perioperative personnel.
Psychological Considerations
During the preoperative period, it is extremely important to identify the children and families who are likely to develop pronounced fear and anxiety before and during the induction of anesthesia. Because the level of stress and underlying temperament that predispose individuals to extreme anxiety may not be overtly apparent, it is essential that the pediatric anesthesiologist carefully evaluate each patient. Indicative behaviors, beyond the obvious crying and uncooperative child, include the absence of social interaction, vocalization, emotional expression, and age-appropriate independence from parents (Kain et al., 1997). It is also helpful to assess family members’ levels of anxiety and coping styles. Premedication with anxiolytics has been shown to be the most consistently effective intervention for facilitating induction and reducing postoperative complications in anxious patients (Bergendahl et al., 2004; Almenrader et al., 2007; Schmidt et al., 2007).
Medical Considerations
Most children and infants scheduled for elective surgical procedures are in good health, but common disorders such as upper respiratory tract infection (URI), reactive airways disease, gastroesophageal reflux, obesity, and hemodynamically stable congenital heart lesions can pose diagnostic and management challenges for the pediatric anesthesiologist. Patients with known medical problems should be carefully interviewed and examined, and if general anesthesia is to be induced, appropriate precautions and interventions should be taken. These issues are explored in detail elsewhere in this text (see Chapters 9, Preoperative Preparation, and 36, Systemic Disorders). An overview of some basic considerations is discussed below.
Upper Respiratory Tract Infections
URI is by far the most common problem the pediatric anesthesiologist encounters, especially in the ambulatory surgery setting. URI and the accompanying inflammation increase upper and lower airway irritability and secretions, and they may increase the incidence of laryngospasm, bronchospasm, and perioperative hypoxemia (see Chapter 36, Systemic Disorders) (DeSoto et al., 1988; Cohen and Cameron, 1991; Coté, 2001; Bordet et al., 2002; Elwood et al., 2003). Risk factors for associated complications include nasal congestion, copious secretions, reactive airways disease, history of prematurity, passive smoking, airway surgery, endotracheal intubation, and laryngeal mask airway insertion (Tait et al., 2001; von Ungern-Sternberg et al., 2007). Even in patients without a history of asthma, airway reactivity can develop with URI or lower respiratory tract infection and last as long as 6 to 8 weeks (Empey et al., 1976; de Kluijver et al., 2002). The decision to postpone the procedure depends on the urgency of the surgery, the severity of symptoms, and the need to instrument the airway (Tait and Malviya, 2005). In these patients, prophylactic bronchodilator treatment should be considered before the induction of anesthesia and before the emergence from anesthesia and extubation.
Reactive Airways Disease
In children with reactive airways disease (RAD), a detailed past medical history must be obtained to determine the severity of the disease and the effectiveness of current medical treatment. Recurrent emergency visits and hospital admissions, especially those to critical care units and/or involving the use of steroids, are red flags for poor control of symptoms. Mild asthma that is poorly controlled may improve with more aggressive treatment, whereas well-controlled severe RAD may require sustained optimized therapy. In a patient with active or recent bronchospasms, elective surgery should be postponed for 4 to 6 weeks. If surgery is required, preinduction treatment with a β2-agonist is recommended to minimize respiratory complications (Scalfaro et al., 2001). Recent steroid use may require perioperative stress-dose steroid coverage (see Chapter 36, Systemic Disorders).
Congenital Heart Disease
Children with congenital heart disease (CHD) may require antibiotic prophylaxis preoperatively for the prevention of bacterial endocarditis. Recommendations by the American Heart Association (AHA) were updated by Wilson et al. in 2007. The recommendations can also be downloaded from the AHA website at http://www.americanheart.org. The guidelines for the AHA subacute bacterial endocarditis (SBE) prophylaxis, which were formulated by an AHA writing group with input from national and international experts, have been significantly modified. CHD conditions associated with the highest risk for SBE are listed as follows:
Prophylaxis is only recommended for “dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa” and “invasive procedure of the respiratory tract that involves incision or biopsy of the respiratory mucosa, such as tonsillectomy and adenoidectomy” (Wilson et al., 2007). Standard antibiotic recommendations include amoxicillin PO or ampicillin IV 50 mg/kg (maximum dose 2 g) 30 to 60 minutes before the procedure. Alternative antibiotics for those patients allergic to penicillin or ampicillin include clindamycin, cefazolin, ceftriaxone, azithromycin, or clarithromycin.
Animal studies indicate that if the preoperative SBE dose is missed, the effective prophylaxis can be given within 2 hours (but not after 4 hours) after the procedure (Berney and Francioli, 1990; Dajani et al., 1997).
Preoperative Fasting
Preoperative fasting times allow for gastric emptying and reduction of aspiration risk. Evidence of rapid gastric emptying in infants and children and efforts to improve the perioperative experience of young patients and their families have resulted in liberalization of pediatric fasting guidelines. Based on clinical observation and studies of residual gastric volumes, international recommendations for NPO times before anesthetic induction in healthy children are 2 hours for clear liquids, 3 to 4 hours for breast milk, 4 hours for infant formula (in infants younger than 3 months), 6 hours for infant formula (in infants older than 6 months), 6 hours for light meals, and 8 hours for heavy meals (Schreiner et al., 1990; Litman et al., 1994; Cook-Sather et al., 2003; Søreide et al., 2005; Murat and Dubois, 2008). This growing consensus has given rise to the 2-4-6 rule. Although these fasting times do not apply to children with gastrointestinal or systemic disorders that may interfere with or slow gastric emptying, Cook-Sather and colleagues (2009) have reported that even in overweight and obese children undergoing elective surgery, the 2-hour minimum preoperative clear-liquid fasting guideline is adequate.
Allowing infants and children to have oral intake closer to the time of surgery can help reduce patient irritability, parental stress, and risk of dehydration. Conversely, the short and multiple fasting times can lead to confusion and NPO violations. It is extremely important that those giving and receiving instruction on preoperative food and fluid intake clearly understand the terminology (i.e., what constitutes a clear liquid), timing, and need for adherence to the guidelines (Schoenfelder et al., 2006). A clear liquid is a solution (as opposed to a suspension) that contains no particulate matter. Examples of clear liquids include water, Pedialyte, carbonated beverages, clear tea, plain gelatin, and fruit juices without pulp (American Society of Anesthesiologists, 1999; Ferrari et al., 1999).
Preanesthetic Preparations
Most children can be well managed in this friendly environment. An anesthesia mask may be given to the child to play with in the waiting area before induction (Fig. 13-1). Allowing children to choose a flavor (e.g., bubble gum, cherry, or grape) can provide them with a sense of control. The additional support of pacifiers, toys, and music boxes is often helpful. Children should keep the objects brought from home, particularly security blankets or other transitional objects, during the induction of anesthesia. Clowns have also been used for the prevention of preoperative anxiety in children (Vagnoli et al., 2005; Golan et al., 2009).
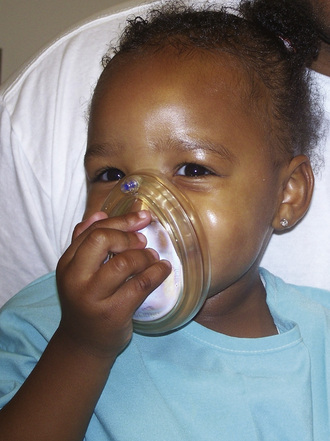
FIGURE 13-1 An anesthesia mask may be given to the child to play with in the waiting area before induction.
Premedication
As stated above, the use of premedication is the most reliably effective intervention for reducing preinduction anxiety and stress for young patients and their parents. Preoperative medications are described in detail in Chapter 9, Preoperative Preparation, and are discussed only briefly here. Historically, premedication referred to long-acting sedatives and anticholinergic agents that were often given intramuscularly (IM) or rectally (PR) to prepare children in inpatients’ units for their trip to the operating room. Contemporary administration of sedatives, typically by mouth, is administered just before induction to facilitate child-parent separation, anesthetic mask acceptance, and/or patient cooperation. IM administration is almost now solely reserved for extremely agitated, uncontrollable children.
Benzodiazepines
Midazolam, a water-soluble benzodiazepine, is the most commonly used preinduction medication (Kain et al., 1997). Given orally at 0.5 mg/kg mixed with fruit-flavored syrup, midazolam will create a calm, euphoric, or drowsy state in most children within 15 to 30 minutes. To potentiate its effect, children should be kept in a nonstimulating environment, and ambulatory children who may become unsteady should be held carefully on the parent’s lap or placed in bed. Coté et al. (2002) showed that a wider range of doses can be effective, adjusting for time of onset, and that respiratory compromise does not occur in otherwise healthy, unmedicated children. Paradoxical reactions, including restlessness, agitation, and disinhibition, occur in approximately 1% to 3% of patients (McMillan et al., 1992; Golparvar et al., 2004). The bitter taste of midazolam, which is difficult to conceal, may reduce acceptance. Midazolam can also be administered via nasal mucosal delivery at 0.2 mg/kg with a more rapid onset but has the disadvantage of an unpleasant burning sensation (Zedie et al., 1996). Prolonged recovery times are not seen with the use of midazolam premedication (Davis et al., 1995b; Bevan et al., 1997). Acetaminophen (20 mg/kg), in fruit-flavored syrup, can also be mixed with midazolam as part of premedication for postoperative analgesia, especially for short ear cases, such as myringotomies (Watcha et al., 1992).
Opioids
The use of opioids for premedication is uncommon in healthy children who are undergoing elective procedures. Even with noninvasive delivery systems such as oral transmucosal fentanyl citrate (OTFC), the advantage of relatively rapid onset is offset by the disadvantages of dysphoria, pruritus, nausea, and vomiting (Goldstein-Dressner, 1991; Ashburn et al., 1993; Epstein et al., 1996). Opioid premedication is best reserved for children experiencing pain, in which analgesia and sedation can be synergistic. The risk of respiratory depression, especially in infants younger than 6 months of age, should always be taken into account.
Ketamine
Ketamine can be given via the oral, nasal, rectal, or IM route (Stewart et al., 1990; Gutstein et al., 1992; Weksler et al., 1993; Tanaka et al., 2000). Ketamine is an effective sedative, but it can also cause increased secretions, nausea, vomiting, psychological disturbances, and prolonged recovery. IM injection of ketamine, 2 to 3 mg/kg (undiluted) may be useful in uncooperative, combative children as the last resort to avoid inhalation induction by force, which increases the risk of physical and psychological trauma to patients (Hannallah and Patel, 1989).
Alpha 2-Adrenergic Receptor Agonists
Oral (4 mcg/kg) and rectal (5 mcg/kg) clonidine can produce excellent perioperative sedation and anxiolysis while reducing the anesthetic requirement, emergence agitation, and postoperative pain, shivering, nausea, and vomiting (Bergendahl et al., 2004; Schmidt et al., 2007; Tazeroualti et al., 2007). The prolonged postoperative sedation seen with clonidine premedication may be advantageous after major surgical procedures but can delay discharge for same-day surgery patients. Similar findings have been described with transmucosal dexmedetomidine 0.5 to 1 mcg/kg (Schmidt et al., 2007; Yuen et al., 2008).
Topical Anesthesia
For children who require or prefer an intravenous (IV) induction, there are multiple approaches to achieving topical anesthesia. Local anesthetics can be delivered without needles through the skin’s protective stratum corneum into the innervated dermal layers via eutectic (EMLA) and liposomal (EL-MAX) creams or driven by mechanisms such as heat, iontophoresis, laser-assistance, or pressurized helium (Hung et al., 1997; Baron et al., 2003; Zempsky et al., 2004; 2008; Sawyer et al., 2009). These techniques, each with limitations, are capable of providing analgesia within 1 to 60 minutes of application. Reported satisfactory anesthesia varies with technique and patient age. Children younger than 6 or 7 years of age tend to report pain, secondary to fear and anticipation of needlesticks, even with apparent anesthesia (Arts et al., 1994; Kleiber et al., 2002).
Parental Presence During Induction
Parental presence during induction of anesthesia (PPIA), in the operating room or a separate area such as an induction room, has increased significantly in the United States over the last decade (Kain et al., 2004). Although the practice avoids separating children from their parents, it has not been shown to decrease patient anxiety or increase cooperation during induction (Kain et al., 1998; Arai et al., 2007). One benefit, which is important in terms of family-centered care, is an increase in parental satisfaction scores. Kain and colleagues, in 2006, confirmed the previous findings of Bevan et al. from 1990 that PPIA had a measurable benefit when a calm parent accompanied an anxious child, and a worsening effect when an anxious parent accompanied a calm child. There was no measurable benefit when both parent and child were calm. Subsequently, they have determined that parental preparation to reduce anxiety (e.g., learning distraction techniques, and avoiding reassuring behavior) can significantly improve the outcome of PPIA (Kain et al., 2007).
Preparation for Induction
Monitoring During Induction
At a minimum, monitoring during induction should include pulse oximetry and capnography. The precordial stethoscope, once the sine qua non of pediatric anesthesia, has apparently been displaced by more accurate and adaptable monitors (Watson and Visram, 2001). However, the precordial stethoscope is still an essential and more sensitive and continuous monitor for changes in breath sounds and the quality of heart beat, especially in infants and young children. Vital signs can vary markedly during the induction of anesthesia and should be observed continuously with a precordial stethoscope and in accordance with the ASA standards for patient safety (1986). If the child is anxious, it is probably best to place additional monitors like ECG pads and a blood pressure cuff after induction instead of losing the opportunity for a calm induction. However, medical condition and early infancy may necessitate full monitoring before and during induction. If this is the case, the baseline measurements should be obtained before the patient is exposed to any anesthetic agent.
Methods of Induction
Inhalation Induction
Once in the operating or induction room, the child may be given the choice to lie down or sit up. If sitting is elected and parents are present, the child can be offered the option to sit in a parent’s lap or next to them (Fig. 13-2). The anesthetic mask, either one introduced in the waiting area or one detached from the anesthesia circuit, should be shown (again) to the child. Putting artificial fruit or candy flavors in the mask may help disguise the odor of the anesthetic. One should never place the mask on the child’s face without warning. Even with preparation, some children, especially those with previous experience with inhalation inductions, may strongly reject the anesthesia mask (Przybylo et al., 2005). The mask may become more acceptable if a family or staff member initially tries it on, or if the anesthesiologist applies it to one of the child’s toys or random body parts before placing it near his or her face. Other methods to introduce the mask include having the child watch the insufflation of the anesthetic bag or hold the mask him or herself as he or she begins to breathe into the circuit. Making a game out of this activity is far better than getting into battle of wills. Distraction techniques such as storytelling, singing, counting, or just talking nonsense are strongly encouraged. If the child still emphatically objects to the mask, other approaches such as supplemental sedation or an IV induction should be considered.
DuBois et al. (1999) compared three induction techniques of conventional tidal-volume breathing of sevoflurane: incremental increasing concentrations (2% to 6% to 8%) in 100% oxygen, 8% in 100% oxygen, and 8% in a 1:1 mixture of nitrous oxide and oxygen. There were minimal differences among the three approaches. Lejus and colleagues (2006) compared the two different breathing techniques of conventional tidal volume and single-breath vital capacity of 7% sevoflurane in children older than 5 years of age. Eyelash-reflex loss was more rapid in the vital-capacity group compared with the tidal-volume group, but the time to deep anesthesia, bispectral index values 60 and 40, and the incidences of side effects were similar in both groups. Of note, the vital-capacity technique was preferred over the tidal-volume technique by the children. The authors propose that the decreased exposure to the smell of sevoflurane and decreased awareness of losing consciousness explained the higher preference scores in the vital-capacity group.
If a child has fallen asleep or is well sedated in a parent’s arms or on a stretcher, anesthesia can be induced by the “steal technique,” as originally described by Guedel in 1921 (Calverley, 1986). While avoiding moving or awakening the child, 70% nitrous oxide at high flows via an anesthesia mask is held closely over the child’s face. At first the mask should not touch the skin, but as sedation deepens it is placed gently on the face while incrementally increasing the concentration of sevoflurane. Monitoring devices should be attached as soon as possible. Once adequately anesthetized, the child can be transferred to a stretcher or operating-room bed if needed.
Maintenance of the Upper-Airway Patency
Upper airway obstruction can arise during induction of anesthesia (even in healthy infants and children) for several reasons, including tongue displacement, excess soft tissue, velopharyngeal collapse, and laryngospasm. Applying pressure to the soft tissue between the rami of the mandible when holding a mask on a patient’s face can push the tongue against the hard and soft palate or displace it posteriorly, leading to occlusion of the oral pharynx or its outlet, respectively. Even minimal relaxation of the pharyngeal and laryngeal muscles during induction can significantly reduce oral and nasal passages already compromised by enlarged or increased amounts of lymphoid tissue. Relaxation of the pharyngeal and laryngeal muscles, accompanied by a marked increase in respiratory effort and excessive generation of negative pressure, which can occur in response to pain and other stimulation, may result in collapse of the velopharynx. To prevent upper airway obstruction from these causes, the pediatric anesthesiologist must learn to hold the anesthesia mask snugly to the patient’s face and perform the triple airway maneuver—neck extension, jaw thrust, and mouth opening—without applying pressure to the soft tissue or causing pain (Fig. 13-3). In addition, a moderate amount of continuous positive airway pressure (CPAP; 10 to 15 cm H2O) can counteract the collapsing force on the relaxed upper airway (Motoyama, 1997; Hammer et al., 2001). Careful and continuous monitoring of breath sounds via a precordical stethoscope is essential to guide and maximize the effectiveness of upper-airway maintenance by hand.
When the patient is sufficiently anesthetized, an oropharyngeal airway may be inserted to further aid in maintaining a patent airway. Insertion of an oral airway in an inadequately anesthetized patient risks triggering laryngospasm. The proper length of an oral airway, with the tip behind the base of the tongue, can be estimated by holding the airway over the side of the child’s face extending from the ear to the angle of the mandible. A preferred method for inserting an oral airway is to slide it gently forward and downward over the tongue while the tongue is pulled outward by a tongue depressor. The technique of inserting the airway upside down and then correcting the orientation in the posterior pharynx should be avoided in children. It tends to push the tongue posteriorly and obstruct the oral pharynx, particularly in infants (Smith, 1980). A nasopharyngeal airway is better tolerated when the anesthesia is too light for insertion of an oropharyngeal airway. This type of airway should be well lubricated and inserted very gently to prevent mucosal injury and bleeding.
If the obstruction is not relieved by airway maneuvers, the patient may have laryngospasm, which can result from laryngeal mucosal irritation and is often initiated by the aspiration of saliva. Vigorous positive pressure ventilation may push the secretions down into the larynx, which intensifies the spasm and further inflates the stomach, compromising pulmonary gas exchange. The risk of regurgitation and aspiration of gastric contents also increases. A healthy child can tolerate a few moments of laryngospasm. A more successful approach is to maintain moderate continuous positive pressure and synchronous ventilation with the “expiratory” phase of laryngospasm, which is when the vocal cords momentarily relax. By using 100% oxygen and intermittent positive pressure, one can ventilate enough gas through the glottis to avoid serious hypoxemia. If rapid oxygen desaturation and bradycardia ensue, IV succinylcholine (2 mg/kg) and atropine (0.02 mg/kg) or 5 mg/kg of IM succinylcholine should be administered without delay (Liu et al., 1981; Hannallah et al., 1986). Airway patency needs to be reestablished with or without tracheal intubation.
Intravenous Induction
Propofol
An induction dose of propofol is 2.5 to 3.0 mg/kg in healthy unpremedicated children between the ages of 3 and 12 years (Manschot et al., 1992). In induction studies, children younger than 2 years of age required a significantly larger dose (2.6 to 3.4 mg/kg), whereas older children needed less (Aun et al., 1992; Manschot et al., 1992). Induction with propofol in children can cause significant decreases in blood pressure and inotropy, similar to those observed after thiopental (Mirakhur, 1988; Hannallah et al., 1991; Manschot et al., 1992).
A major drawback to propofol is the pain it causes on injection. In a quantitative systematic review, Picard and Tramèr (2000) determined that the only approach that had a reliably low number of needed-to-be-treated (NNT) patients was the lidocaine-tourniquet technique (similar to a Bier block; see Chapter 16, Regional Anesthesia) and that preinjection of local lidocaine and analgesics, temperature of medication, speed of infusion, and vein size had no significant effect. Yew et al. (2005) and Rochette et al. (2008) have shown that the addition of medium-chain triglyceride to propofol preparation also decreases pain on injection. Propofol offers the advantage of having antiemetic properties (Borgeat et al., 1990).
Thiopental
The induction dose of thiopental in healthy children is 5 to 6 mg/kg (Coté et al., 1981). In infants between 1 and 6 months of age, the median effective dose (ED50) is reported to be 6.8 mg/kg; in infants younger than 2 weeks of age, it is 3.4 mg/kg (Jonmarker et al., 1987). The dose for induction in infants younger than 5 months was almost twice that for older children (see Chapter 7, Pharmacology of Pediatric Anesthesia, Fig. 7-18). Like propofol, thiopental is a cardiac depressant and vasodilator, and it should be used with care in patients suspected to have hypovolemia or decreased cardiac function.
Etomidate
The induction dose of etomidate in healthy children is 0.3 mg/kg, and it is often recommended in patients who have limited hemodynamic reserve. Sarkar and colleagues (2005) confirmed that in children there are no significant changes in right atrial, aortic, or pulmonary artery pressure, or systemic or pulmonary vascular resistance after bolus dosing. Side effects include pain on injection and myoclonic movements.
Maintenance of anesthesia
Pharmacologic Agents
Inhaled Anesthetics
Inhaled anesthetics are the most commonly used agents in the administration of general anesthesia in pediatric patients. Although halothane was a staple in pediatric anesthesia for years, the development of newer agents with better safety profiles has made halothane nearly obsolete (Lerman, 2004). With discontinuation of the production of halothane in the United States, sevoflurane, desflurane, and isoflurane are now the most commonly used inhalation agents. Nitrous oxide is also commonly used as an adjunct, although there remains some controversy regarding its use.
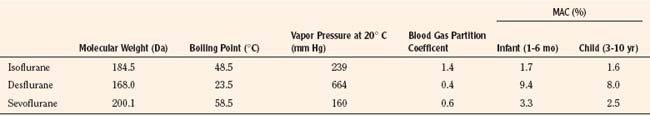
Introduced in 1995, sevoflurane became the agent of choice for inhalation inductions. In addition to its rapid and smooth induction of anesthesia, sevoflurane causes few arrhythmias, less cardiovascular depression, and minimal renal or hepatic toxicities, all of which are improvements over halothane (Lerman, 2004). Isoflurane and desflurane also have more stable cardiovascular profiles and less associated toxicity compared with halothane. However, each agent has limitations that warrant consideration in certain patients and clinical situations. Table 13-1 summarizes the characteristics of the commonly used volatile anesthetics.
Nitrous Oxide
Nitrous oxide is the oldest and has been the most widely used anesthetic in both adults and children. It has an inoffensive odor and a very low solubility (blood-gas partition coefficient of 0.47) that results in rapid uptake and distribution. Because it has a minimum alveolar concentration of 104% for general anesthesia, nitrous oxide cannot be used as a sole anesthetic at normal atmospheric pressures. Therefore, it is generally used as an adjunct to reduce the minimum alveolar concentration of a primary inhalation agent, increasing the rate of induction of general anesthesia, as well as providing or augmenting the analgesic aspect of general anesthesia (Emmanouil and Quock, 2007).
Nitrous oxide has mechanisms of action that continue to be discovered. It appears that the anesthetic effect occurs through inhibition of N-methyl-d-aspartate (NMDA) glutamate receptors in the central nervous system. Analgesia occurs through release of endogenous opioids that then stimulate opioid receptors and spinal level γ-aminobutyric acid (GABA) receptors. Anxiolysis occurs through activation of GABAA, although this pathway is still being investigated (Emmanouil and Quock, 2007).
Although nitrous oxide is widely used, there are certain patients and clinical situations in which its use is not recommended and even contraindicated. In adults with pulmonary hypertension, nitrous oxide increases pulmonary artery and pulmonary wedge pressures (Schulte-Sasse et al., 1982). However, in healthy infants, Hickey and colleagues (1986) observed mild decreases in heart rate, blood pressure, and cardiac index with no increase in pulmonary artery pressure or vascular resistance.
Nitrous oxide accumulates in closed, gas-containing spaces and should be avoided in patients at risk of toxicity caused by this expansion. This includes patients with obstructed loops of bowel, pneumothorax, pneumocephalus, and middle-ear surgery. Nitrous oxide also has been shown to increase middle cerebral-artery blood-flow velocity and may increase intracranial pressure (ICP), making its use in children with increased ICP contraindicated (Wilson-Smith et al., 2003). Furthermore, in situations in which maximal oxygen delivery is needed, such as shock, massive blood loss, severe anemia, and compromised cerebrospinal blood flow, nitrous oxide should be avoided. Children with abnormal vitamin B12 and B12-related metabolism may also be at risk of neurologic injury after exposure to nitrous oxide during a routine anesthetic procedure (Sanders et al., 2008).
The role of nitrous oxide in postoperative nausea and vomiting (PONV) has been examined extensively. Although the incidence was increased when used with propofol, no difference was seen in the incidence of PONV with or without nitrous oxide when used with sevoflurane or desflurane in children (Watcha et al., 1991; Kuhn et al., 1999; Bortone et al., 2002). Nitrous oxide supports combustion. Thus, its use in patients undergoing oral or facial procedures should be reevaluated with regard to the possibility of an airway fire.
Sevoflurane
Since its introduction in the mid 1990s, sevoflurane has replaced halothane as the agent of choice for inhalation inductions in pediatric patients. It is a fluorinated methyl isopropyl ether with a blood-gas partition coefficient of 0.68, allowing for rapid induction and recovery. With a nonpungent odor and minimal airway irritation, up to 8% sevoflurane can be delivered without significant breath holding, coughing, or laryngospasm. The minimum alveolar concentration (MAC) of sevoflurane decreases from 3.3% in neonates and infants younger than 6 months old to 2.5% in children between 6 months and 5 years old and to 2.0% in adults (Hatch, 1999). Lerman and colleagues (1994) found that the MAC-sparing effect of nitrous oxide was significantly less for sevoflurane.
Sevoflurane causes minimal cardiovascular side effects in children. Compared with desflurane, sevoflurane produces less hypotension. There is also less tachycardia than isoflurane and less myocardial depression than halothane (Frink et al., 1992b; Holzman et al., 1996). Using echocardiography, Wodey et al. (1997) found that sevoflurane did not affect heart rate, cardiac index, or myocardial contractility. Furthermore, it does not sensitize the myocardium to epinephrine (Hayashi et al., 1987). Arrhythmias are uncommon with sevoflurane compared with other agents (Hatch, 1999).
Sevoflurane affects respiratory function, with some studies suggesting it does so to a greater extent than other agents (Doi et al., 1994; Yamakage et al., 1994). Brown and colleagues (1998) found that compared with halothane, minute ventilation and respiratory frequency were lower in infants on 1 MAC of sevoflurane, but that there were only moderately increased end-tidal carbon dioxide (CO2) levels. Sevoflurane is, however, an effective bronchodilator (May et al., 1996). Neurologically, sevoflurane has been associated with cortical epileptiform electroencephalograms, although no lasting clinical sequelae (such as seizures) have been attributed to sevoflurane alone. Further studies are necessary to determine which patients are at risk and what can be done to decrease the incidence of these EEG changes (Constant et al., 2005).
As much as 5% of sevoflurane is metabolized, with defluorination producing an inorganic fluoride that is then excreted in the urine. Peak fluoride concentrations, although higher in sevoflurane than in isoflurane, are still well below the accepted nephrotoxic level of 50 mmol/L. There is rapid elimination, and these levels remain below toxic levels even with prolonged anesthetics (Levine et al., 1996; Hatch, 1999). In addition, the metabolism of sevoflurane by the P450E1 system is mostly in the liver and not the kidneys. Thus, the concern of nephrogenic diabetes insipidus (DI) by elevated fluoride levels does not appear to be an issue.
Sevoflurane reacts with soda lime in the anesthesia circuit, resulting in the production of compound A. Compound A increases more when Baralyme brand (Allied Healthcare Products Inc., St. Louis, Mo) is used, when dry rather than wet absorbents are used, and when lower fresh gas flows (0.5 to 1 L) are used, resulting in increased canister temperatures (Frink et al., 1992a). Ebert and colleagues did not find evidence of renal injury after sevoflurane was administered in high concentrations (3% end-tidal) for 8 hours (1998). Kharasch and colleagues (1997) also found no significant renal dysfunction in patients with low-flow sevoflurane (1 L/min) when compound A was detected. Other studies have also documented no worsening of renal function after sevoflurane, even in patients with preexisting renal impairment (Conzen et al., 1995). New generation CO2 absorbers, including DragerSorb Free and Amsorb Plus, have been shown to provide adequate CO2 absorption while reducing the production of compound A, even at fresh gas flows as low as 500 mL/min (Marini et al., 2007).
Spontaneous ignition, fire, and explosion resulting from an exothermic reaction of sevoflurane with desiccated CO2 absorbers have also been reported (Castro et al., 2004; Wu et al., 2004). With high fresh gas flow, the increasing dryness of the absorbent increases degradation of sevoflurane. Dunning et al. (2007) demonstrated that up to 3 mol of hydrogen were produced in the reaction of sevoflurane with heated, desiccated absorbent. The authors postulate that this hydrogen is the most likely fuel in anesthesia machine fires. Special attention should be paid to situations in which absorbent desiccation may be greater, such as in seldom-used anesthesia machines and in operating rooms where high fresh-gas flows relative to body size are used (Wu et al., 2004). The use of newer absorbents or Mapleson D circuits can also help avoid this problem (Woehlck, 2004).
While the question of sevoflurane-related hepatitis has been raised in case reports, metabolism of sevoflurane does not result in the trifluoroacetylated liver proteins that trigger the immune-mediated hepatitis seen with halothane, and immune-based hepatitis after sevoflurane has not been reported (Kharasch, 1995; 2008). Malignant hyperthermia has been reported with sevoflurane use (Otsuka et al., 1991).
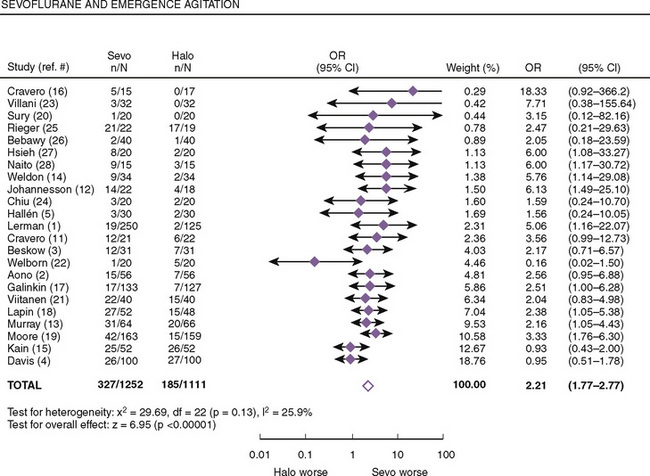
Both postanesthetic emergence time and the time when discharge criteria are met are significantly lower in patients given sevoflurane compared with those receiving halothane; however, patients receiving sevoflurane had significantly higher pain scores that required analgesics to be administered earlier (Naito et al., 1991; Sarner et al., 1995). In a meta-analysis of randomized controlled trials, Kuratani and Oi (2008) demonstrated that sevoflurane had a higher probability of emergence agitation compared with halothane (Fig. 13-4). Treatment or pretreatment with either dexmedetomidine, fentanyl, or propofol can mitigate emergence agitation after sevoflurane anesthesia.
Desflurane
Desflurane is a fluorinated methyl ethyl ether with a blood:gas solubility coefficient of 0.42, which is close to nitrous oxide and lower than any other agent. With lower potency, the MAC in newborns is 9.2%, in infants it is 8% to 9.9%, and in older children and adults it is 6% (Hatch, 1999). Because of its lower boiling point (22.8° C), desflurane requires a heated pressurized vaporizer. It is also resistant to degradation and biotransformation.
Desflurane is highly irritating to the upper airway. Zwass and colleagues (1992)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree