(1)
Trauma and Critical Care, R Adams Cowley Shock Trauma Center, Baltimore, MD, USA
Keywords
Axillary artery exposureBrachial artery exposureInferior vena cava exposureIntra-abdominal aorta exposureIliac vessel exposureFemoral artery exposurePopliteal artery exposureVascular injuryVascular exposureShuntFasciotomyHistory of Care
Patients who present with hemorrhagic shock are assumed to have a vascular injury until proven otherwise. As with all patients, tenants of ATLS should be followed. Obvious external hemorrhage should be immediately controlled with direct pressure, tourniquets, or clamps.
Locations for hemorrhage include the chest, abdomen, pelvis, long bones, and external loss. Hard signs and symptoms that are highly diagnostic of vascular injury include active/pulsatile bleeding, shock not explained by other injuries, expanding or pulsatile hematoma, absent peripheral pulse, audible bruit, palpable thrill, or evidence of regional ischemia (pain, pallor, paresthesia, paralysis, pulselessness). While care must be individualized, in general, patients with hard signs of vascular injury require emergent operative control and repair. Soft signs and symptoms that are suggestive but not diagnostic of vascular trauma include mild shock, stable hematoma, slow bleeding, injury in proximity to a major neurovascular tract, peripheral nerve injury, and diminished pulses. Patients with soft signs or orthopedic injuries associated with potential vascular injuries should undergo diagnostic testing. Figure 5.1 shows the Western Trauma Association (WTA) algorithm for the workup of peripheral vascular injury.
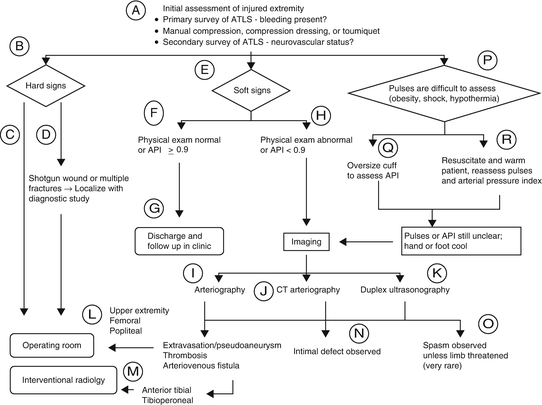

Fig. 5.1
The WTA developed an algorithm for the workup of peripheral vascular injury.
For injured extremities, the injured extremity index should be obtained. Documentation of the Doppler determined arterial pressure in an injured extremity, divided by the pressure of an unaffected limb using a manual blood pressure cuff provides the value. An index above 0.9 excludes injury, while a value of less than 0.9 warrants further investigation such as CTA, angiogram, or exploration [2, 3].
Assessing distal pulses may be difficult in a patient in shock or who is hypothermic; therefore, these patients should be resuscitated in the usual fashion, and once improved, palpation and distal assessment with noninvasive testing should be performed [1].
In addition to CT angiogram and formal angiogram, both MR angiogram and duplex have been advocated for use. There is such an array of options, and some of these are operator dependent. The imaging modality of choice is currently site specific. The multicenter AAST prospective observational vascular injury trial (PROOVIT) is prospectively collecting data regarding imaging, for further guidance.
In combined long-bone fractures and vascular injury, immediate involvement of orthopedics should occur in conjunction with the trauma team. To maximize limb salvage, the interval between injury and reperfusion should be minimized to less than 6 h. Restoration of flow should take priority over skeletal injury management by shunting if there is an unstable fracture or dislocation or by definitive arterial repair if the skeletal injury is stable and not significantly displaced. If a shunt is used, after the extremity is stable, the formal repair can take place at that time or once the patient is clinicaly stable. Combined arterial and skeletal trauma pose a high risk for compartment syndrome so fasciotomies are strongly considered [4]. In patients who are unable to be examined, such as head injured patients or patients intubated for other reasons, I almost always perform fasciotomies since clinical exam can be inaccurate and you lose the subjective aspects for the diagnosis. Serial compartment pressures are time consuming and compartments can be missed on evaluation.
Venous injuries encountered during exploration for arterial injuries should be repaired if the patient is hemodynamically stable and the repair will not significantly delay the treatment of associated injuries, otherwise ligation is an option. Suprahepatic IVC, superior mesenteric vein, and portal vein injuries must all be repaired as ligation would be fatal. The highest patency rates are achieved with lateral venorrhaphies that do not significantly narrow the lumen of the repaired vein. For complex repairs, end-to-end or paneled repairs are likely the best options. Synthetic and interposition repairs have the worst reported patency rates. Regardless of the repair, lower extremity edema and thrombosis rates are high [4]. With artery and vein injury of the lower extremity, especially with vein ligation, fasciotomies should be performed.
Fasciotomies and Compartment Pressures
Patients that are “high risk” for developing early compartment syndrome after trauma include hypotension in the field; delay in treatment, especially without arterial inflow for 4–6 h; ongoing hypotension during resuscitation or operation; evidence of crush injury combination of arterial and venous injury, especially the popliteal artery and vein; and need for arterial or venous ligation or early thrombosis and repair of either [5]. Compartment pressures can be measured intraoperatively or postoperatively if a fasciotomy was not performed. Devices such as the Stryker Intra-Compartmental Pressure Monitor System (Stryker Instruments, Kalamazoo, MI) or arterial line with slit/side port catheter can be used. There is ongoing debate about absolute pressures versus delta P (compartment perfusion pressure (CPP) = mean arterial pressure − compartment pressure) on who needs a fasciotomy. Absolute pressures of 30–35 mmHg or delta P of less than 30 mmHg should warrant fasciotomy [5].
Technique with Personal Tips
In the emergency department, foley balloon tamponade can be used in the neck/upper chest for noncompressible hemorrhage during transition to the OR. Saline is used to inflate the balloon and a clamp is placed on the other distal portion of the catheter so blood does not continue to flow out the catheter. In the operating room, an angiographic compatible bed is key for identifying an injury or for intraoperative imaging during the case. Loupe magnification and headlamps are also very helpful for taking care of these injuries.
The affected area should be widely prepped and draped for adequate exposure. This prep should include proximal areas outside of the zone of injury, in case vascular control must be gained at that location. With patients that have neck or upper extremity vascular injury, the chest should be included, in case a sternotomy, thoracotomy, or a clavicular incision is needed for more proximal control. With patients that have lower extremity injury, the abdomen should be included in the prep, in case access is needed to the external iliac arteries. If manual pressure is required to maintain hemostasis, the hand should be prepped into the field until another source of control can be obtained. The lower extremity, groin, and thigh should always be included, as saphenous vein from an uninjured leg may be needed as conduit.
Proximal and distal control of the injured vessel is the basic principle of vascular surgery. If the injury is to an extremity and it is distal to the groin or axilla, a sterile pneumatic tourniquet can serve as inflow control, until the vessel can be properly dissected out. Once identified, control can be obtained with vascular clamps, bulldogs, or vessel loops and the tourniquet taken down. For the upper extremity, proximal vascular control can be obtained on the brachial artery, axillary artery, subclavian artery, or great vessel control via sternotomy, depending on the location of the injury. For the lower extremity, superficial femoral artery, common femoral artery, or external iliac artery control may be needed, depending on the location of the injury. The external iliac can be accessed either transperitoneally or through a lower abdominal oblique incision into the preperitoneal space, like in transplant surgery. Distal control should also be performed outside the area of hematoma or hemorrhage. If this is difficult, direct control can be performed and internal balloon tamponade with a fogarty and a three-way stopcock can be used. Wherever the injury is located, using clamps far proximally and distally and then walking them in towards the wound can provide excellent control and avoid extra hemorrhage rather than trying to attack these wounds in close proximity to the injury.
Once the vessel is isolated both proximally and distally, vessel loops are used for inflow and outflow control. The injury should be assessed as to whether a simple or complex repair is needed. Additional injuries and the patient’s hemodynamic status must be considered. In the case of complex injury and/or if the patient is unstable, damage control with shunting or ligation should be used (see shunt section).
The vessel should have the edges debrided to normal, healthy tissue. Inflow and back bleeding should be noted. A fogarty catheter can be run proximally and distally to clear the vessel of any thrombus. Distally, the catheter should be used until no thrombus is returned on two consecutive passes. Appropriate sizes for the catheter include a #6 for the common and external iliac arteries, a #4 to #5 for the common femoral artery, a #4 for the superficial femoral artery, a #3 to #4 for the popliteal artery, and a #3 for the other arteries of the leg. Balloon catheters are never passed in venous injuries because these will disrupt the veins [6].
Systemic heparinization should be considered in stable patients with isolated extremity/vascular injury. 5,000 (50–75 units/kg) units IV should be given. Systemic heparin should be avoided in patients with torso or head injuries. Local administration of heparinized saline should be injected both proximally and distally before any repair to aid in preventing local thrombosis using approximately 20–25 mL per site (50 U/mL).
If the injury to the artery only has minimal loss, a primary repair can be used depending on the luminal diameter. When closing, the last stitch or two is left loose until proximal and distal flushing are performed. Care is made not to cinch down the last knot or knots too tightly to avoid constricting the anastomosis. For partial arterial injuries of smaller vessels (brachial, SFA, popliteal), patch angioplasty can be used so the resulting luminal diameter is not too narrow.
For a complete transection, the injured vessel must be debrided to healthy tissue at both ends. Some length can be gained by sacrificing some of the branches of the vessel depending on its location. This maneuver may gain up to 3 cm of total advancement of both ends. If this is not feasible or the defect is too large, an interposition graft is necessary [6]. Choice of conduit will depend on size match and location of the injured vessel. Greater saphenous vein from the uninjured leg is typically the first choice for conduit. As the injured artery is typically vasoconstricted, consider a slightly larger conduit in what is visualized.
For suturing into the arterial wall, the key maneuver is perpendicular passes of the needle. Most vascular trauma is to young healthy arteries, so although formal vascular training teaches “inside out” on the artery to prevent intimal flaps, this is less worrisome in vascular trauma. For the repair, it is easier to perform the distal anastomosis first, especially if it is in a difficult location to allow for better visualization of the posterior suture line. Figure 5.2 shows the WTA algorithm for operative decison making in vascular injury.
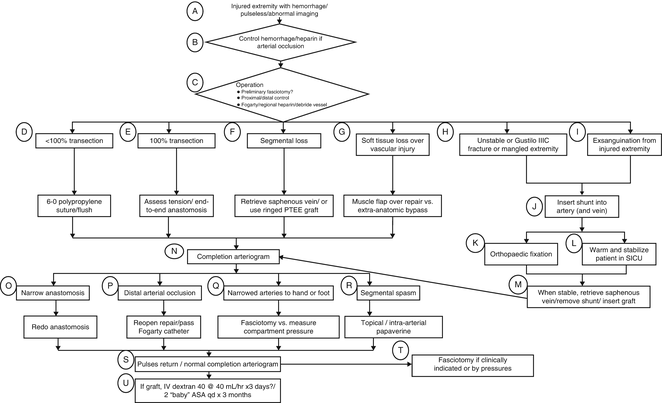

Fig. 5.2
The WTA II flow chart shows one algorithm for the operative decision making for vascular injury.
Saphenous Vein
The ideal conduit for extremity vascular injury is contralateral saphenous vein. The saphenous vein can be used as the primary conduit, patch, or spiraled for reconstruction of larger vessels. The amount of vein harvested should be longer than the defect as it may be trimmed down prior to anastomosis. During vein harvest, small branches should be ligated with 2-0 or 3-0 silk. After the vein is harvested, it should be reversed and an olive-tip catheter secured to the distal end with silk suture. Using heparinized saline, the vein should be dilated while closing the other end to look for any leaks. These leaks can be controlled with ties or small Prolene sutures. One side of the vein is marked using a sterile marker, so as to not twist the conduit when performing the anastomosis. Once the conduit is satisfactory, it is placed in a heparinized saline bath. If the greater saphenous vein is absent, diseased, has a poor diameter, or is the only venous outflow for an injured extremity, other options include the lesser saphenous or the cephalic or brachial vein [6].
PTFE
Due to the size of injured vessels ideally, PTFE should only be placed proximally to the axilla and/or to the knee. If the injury is distal to this location, vein should be used because the diameter of the injured vessel is small and smaller PTFE will clot. PTFE has shown improved patency (70–90 % short term) and rare infection, even in contaminated wounds [7, 8]. It is clear that patency with PTFE is equivalent to that of a vein for injuries proximal to the popliteal artery; PTFE grafts smaller than 6 mm should not be used. 8 mm grafts are typically the smallest I would use. PTFE and vein grafts must be covered with soft tissue or there is a significant risk of hemorrhage from desiccation of the vein, with subsequent autolysis or breakdown of the anastomosis [9]. Patients with PTFE placed should be put on ASA of 162–325 mg daily × 3 months postoperatively [10]. The ASA recommendation is extrapolated from aortosaphenous bypass from CABG data as well as from bypass from peripheral vascular disease.
Other Conduits
Other conduits for vascular repair include bovine pericardium as well as CryoVein. As these are tissue conduits, they may be better options than PTFE if there is poor autologous saphenous vein for conduit or a size mismatch. They can also be used in case of vascular graft infection. There is little published data on these techniques and none in the trauma literature.
As with PTFE, healthy tissue must be used to cover the repair. Local muscular flaps may be used. In an unstable patient without time for complicated coverage, porcine xenograft or other biologic can be placed under mesh gauze soaked with antibiotics [6].
Shunts
Shunts are the primary damage control modality for vascular injury. In an unstable situation, a shunt can be placed to re-establish blood flow and the patient can be resuscitated in the OR or ICU. A formal repair can be performed when the patient is stable. Various commercial shunts are available in assorted sizes and configurations. If commercial shunts are not available, anything from IV tubing to pediatric feeding tubes all the way to chest tubes can be used for conduits, depending on the size of the injured vessel. We use Argyle shunts because they are straight, providing direct flow. The shunt size should be the largest diameter that will fit comfortably into the injured vessel. Other shunts can loop and become dislodged so we typically do not use them. Venous shunting can be considered depending on location; however, in true exsanguination, ligation may be used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








