Immunizations
Camille Sabella
The goal of immunization is to prevent infectious diseases. In the United States, immunization programs have resulted in the elimination or significantly diminished incidence of many infectious diseases. The implementation of universal immunization against certain infections, such as measles, rubella, and infection with Haemophilus influenzae Type b (Hib), has resulted in a decrease of more than 99% in the prevalence of these infections.
Active immunization involves administering all or part of a microorganism or a modified product of such virulence to evoke an immunologic response. This response mimics the response to natural infection but poses little or no risk to the recipient. Active immunization can be accomplished with either live attenuated vaccines or killed (inactivated) vaccines. Vaccination with a live vaccine causes an active infection in the vaccinee but usually results in little or no adverse host reaction in an immunocompetent person. An immunocompromised person, however, may experience an adverse effect after vaccination with a live virus vaccine. The microorganisms in inactivated vaccines are incapable of replicating in the host and therefore do not pose a threat to an immunosuppressed host.
Passive immunization involves the administration of preformed antibody. This can be given for the following reasons:
Prevention or amelioration of an infection in a susceptible host who has been exposed to the infection and is at high risk for its complications. A clinical example would be the administration of varicella-zoster immunoglobulin (Ig) to a susceptible immunocompromised host who has been exposed to varicella.
Amelioration or provision of aid in suppressing the effect of the toxin when an infection is already present. A clinical example would be administration of tetanus Ig to treat a patient with tetanus.
Replenishment of Ig in a person with deficient antibody synthesis because of a congenital or acquired B-cell defect. A clinical example would be administration of intravenous Ig monthly to a child with Bruton (X-linked) agammaglobulinemia.
GENERAL CONSIDERATIONS
In general, multiple vaccines can be administered simultaneously in a safe and effective manner. The immune response to one of the vaccines included in the routine immunization schedules generally does not interfere with the immune response to the others. A causal relationship between multiple immunizations and the development of immune system dysfunction has recently been rejected by an Institute of Medicine Safety Review Committee.
The recommended doses of vaccines are based on clinical trials and experience. Administering a reduced dose of a vaccine may result in a suboptimal immunogenic response to the vaccine. Therefore, reducing or dividing the dose of a vaccine is not recommended, even in premature or lowbirth-weight infants.
The administration of parenteral live virus vaccines shortly before to several months after the administration of Ig products can result in diminished immunogenicity. This phenomenon has been documented for measles vaccine and theorized for varicella vaccine. The degree and duration of inhibition of the immune response vary with the dose and route of administration of the Ig product. If an Ig product has been administered within 14 days of the administration of measles, mumps, and rubella (MMR) or varicella vaccine, the vaccine should be readministered after a period deemed appropriate, depending on the dose and type of Ig given. If a child has received an Ig product and is due to receive an MMR or varicella vaccination, the vaccination should be deferred until an appropriate amount of time has elapsed. The intervals between the administration of select Ig products and the vaccination with measles and varicella vaccines recommended by the Committee on Infectious Diseases of the American Academy of Pediatrics are summarized in Table 2.1.
The National Childhood Vaccine Injury Act of 1986 requires health care professionals who administer routine vaccines to:
Maintain permanent immunization records
Report occurrences of specified adverse events to the Vaccine Adverse Event Reporting System (VAERS)
TABLE 2.1 RECOMMENDED TIME INTERVALS BETWEEN ADMINISTRATION OF AN IMMUNOGLOBULIN PRODUCT AND IMMUNIZATION WITH MEASLES OR VARICELLA VACCINES | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
CONTRAINDICATIONS TO VACCINATION
Contraindications to vaccination are discussed in the subsequent text in the sections on specific vaccines. In general, if an infant or child has an acute febrile illness, unless it is mild with only low-grade fever, immunizations should be deferred until recovery. An otherwise healthy child who is on antimicrobial therapy, has a mild diarrheal illness, or recently has been exposed to an infectious disease can be vaccinated. Likewise, an otherwise healthy child who has a pregnant, unimmunized, or immunodeficient household contact can be vaccinated. Breast-feeding, malnutrition, and a family history of seizures or adverse events after immunizations are not contraindications to immunizations.
Premature infants generally can be immunized at the usual chronologic age. The one possible exception concerns hepatitis B immunization in infants weighing less than 2 kg. These infants vaccinated with hepatitis B vaccine at birth have seroconversion rates after hepatitis B vaccination that are lower than those in term infants and in preterm infants vaccinated at a later date. However, medically stable preterm neonates appear to respond to hepatitis B vaccination as well as older infants. Thus the following recommendations apply for hepatitis B vaccination of infants weighing less than 2 kg.
If the mother is hepatitis B surface antigen (HBsAg)-negative:
Defer the first dose of hepatitis B vaccine until the infant is 30 days of age if medically stable or at hospital discharge if the infant is discharged before 30 days of chronologic age. The second and third doses of vaccine can then be administered at 1 to 2 months and 6 to 18 months of age, respectively.
If the mother is HBsAg-positive:
Administer the first dose of hepatitis B vaccine to the infant within 12 hours of birth, along with hepatitis B Ig.
Complete the immunization series with three subsequent doses of hepatitis B vaccine (do not count the first dose given as part of the immunization series), when the infant is 1 month, 2 to 3 months, and 6 to 7 months of age.
Test the infant for the presence of HBsAg and antibody to HBsAg (anti-HBs) at 9 to 18 months of age.
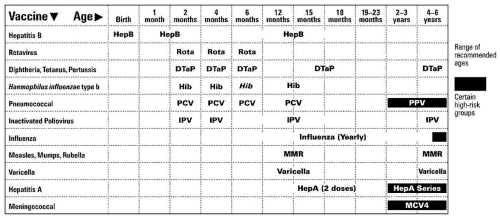
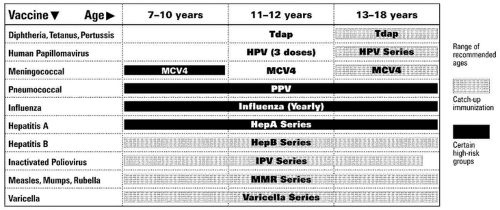
ACTIVE IMMUNIZATIONS ROUTINELY RECOMMENDED FOR ALL CHILDREN (Figs. 2.1 and 2.2)
Tetanus Vaccine
Tetanus vaccine is a killed toxin (toxoid) vaccine that is usually combined with diphtheria and acellular pertussis vaccine (DTaP and Tdap) or with diphtheria vaccine (DT or Td). Vaccination with a primary series and booster doses every 10 years is nearly 100% effective in preventing tetanus. Natural disease usually does not confer immunity to tetanus.
After the primary series is complete, booster doses should be given every 10 years. Booster doses should not be given more frequently than every 10 years except for tetanus postexposure wound prophylaxis (see subsequent text).
Adverse effects include extremely rare reports of severe anaphylactic reactions, Guillain-Barré syndrome, and brachial neuritis. The only contraindication to tetanus vaccination is an immediate anaphylactic reaction to a previous dose of tetanus-containing vaccine. In this rare circumstance, referral to an allergist and possible desensitization is warranted.
Tetanus Prophylaxis in Wound Management
For children who have received three or more doses of tetanus toxoid previously, tetanus vaccination is recommended if the time elapsed since the last dose of vaccine is 5 years (for contaminated wounds) or 10 years (for clean, minor wounds). For children who have received no or fewer than three doses of tetanus vaccine, this vaccine, along with tetanus Ig, should be administered for contaminated wounds, whereas only tetanus vaccine should be administered for clean, minor wounds. The choice of tetanus vaccine depends on the age of the child and whether pertussis vaccine is contraindicated:
For children younger than 7 years, DTaP is recommended unless pertussis vaccine is contraindicated, in which case DT should be given.
For children 7 to 10 years of age or older, adult-type diphtheria vaccine should be administered in combination with tetanus vaccine (Td).
For adolescents 11 to 18 years of age, Tdap should be administered instead of Td, unless they have received Tdap previously.
Diphtheria Vaccine
Diphtheria vaccine is a killed toxin (toxoid) vaccine given in combination with tetanus toxoid and acellular pertussis
vaccine (DTaP or Tdap) or with tetanus toxoid (DT or Td). DTaP and DT should be used for infants and children younger than 7 years, Td should be given to children 7 to 10 years of age, and Tdap used in adolescents 11 to 18 years of age. The dose of diphtheria toxoid in the Td and Tdap preparations are significantly lower than that in DTaP and DT and are therefore less reactogenic in older children and adults.
vaccine (DTaP or Tdap) or with tetanus toxoid (DT or Td). DTaP and DT should be used for infants and children younger than 7 years, Td should be given to children 7 to 10 years of age, and Tdap used in adolescents 11 to 18 years of age. The dose of diphtheria toxoid in the Td and Tdap preparations are significantly lower than that in DTaP and DT and are therefore less reactogenic in older children and adults.
Immunization with diphtheria toxoid is effective in preventing infection and decreasing colonization by toxinogenic strains of Corynebacterium diphtheriae, as evidenced by the rarity of this disease in countries with high immunization rates.
Adverse reactions to diphtheria toxoid include mild local reactions, such as tenderness, swelling, and erythema. In children 7 years of age and older, the incidence of local
reactions to the standard diphtheria dose contained in DTaP and DT is higher; therefore, they should be immunized with Td or Tdap. Anaphylaxis is an extraordinarily rare event following diphtheria and tetanus vaccination.
reactions to the standard diphtheria dose contained in DTaP and DT is higher; therefore, they should be immunized with Td or Tdap. Anaphylaxis is an extraordinarily rare event following diphtheria and tetanus vaccination.
The only contraindication to diphtheria vaccination is a history of an immediate severe or life-threatening event after a previous dose of diphtheria vaccine.
Pertussis Vaccines
Whole-cell vaccines containing inactivated Bordetella pertussis organisms have been replaced by acellular vaccines for routine immunization in the United States. Acellular vaccines contain one or more purified antigens of the bacteria and are combined with diphtheria and tetanus toxoids. Acellular vaccines cause fewer reactions than wholecell vaccines.
The immunogenicity and efficacy of acellular vaccines are comparable with those of whole-cell vaccines. The efficacy of these vaccines in preventing pertussis following primary vaccination is between 70% and 85%.
Acellular pertussis vaccines are recommended for infants and children in the United States because they are associated with fewer adverse events, such as fever, irritability, and local reactions, than whole-cell vaccines. Pertussis vaccines are not currently indicated for children 7 to 10 years of age, although Tdap vaccines are now approved for use in adolescents 11 to 18 years of age.
Adverse events following pertussis vaccination include the following:
Erythema, swelling, and pain at the injection site
Fever, usually low-grade, very rarely to 40.5°C (104.9°F) or higher
Anaphylaxis and allergic reactions
Seizures, most of which are febrile seizures
Hypotonic-hyporesponsive episodes
Persistent, inconsolable crying
Limb swelling
The incidence of systemic and local reactions is significantly less following acellular pertussis vaccination than following whole-cell vaccination. The seizures that follow the administration of pertussis vaccines are thought to be mostly febrile seizures and are not associated with long-term sequelae. Likewise, the hypotonic and prolonged episodes of crying do not appear to be associated with any sequelae. Limb swelling involving the entire thigh or upper arm, sometimes occurring with fever, erythema, and pain, has been reported to occur after booster doses of acellular pertussis vaccines. This condition resolves spontaneously and without sequelae.
Examination of occurrences of sudden infant death syndrome, brain damage, seizure disorder, and developmental delay following pertussis vaccination has not established a causal relationship.
Contraindications to pertussis vaccination include the following:
Immediate anaphylactic reaction to a previous dose of the vaccine
Severe encephalopathy occurring within 7 days after a previous dose of a vaccine that cannot be explained by another cause
Adverse events associated with pertussis vaccination, such as seizures within 3 days of after vaccination, inconsolable crying within 3 hours of vaccination, a hypotonic episode within 48 hours of vaccination, and fever to 40.5°C (104.9°F) or higher, are not considered true contraindications to further vaccination. However, in these situations, the benefits and risks should be carefully considered before vaccination.
The decision to administer pertussis vaccines to children with underlying neurologic disorders must be considered carefully and on an individual basis. In general, in children with progressive, unstable, or evolving neurologic disorders, including recent or uncontrolled seizures, pertussis vaccination should be deferred until the condition has been stabilized. Children with stable neurologic conditions or well-controlled seizure disorders may be immunized with DTaP. A family history of seizure disorder is not a contraindication to immunizing a child with pertussis vaccine.
Poliovirus Vaccines
Poliovirus vaccination, which began with the introduction of the inactivated poliovirus vaccine (IPV) in the 1950s, followed by the oral poliovirus vaccine (OPV) in the 1960s, has virtually eliminated paralytic poliomyelitis worldwide. The last indigenously acquired case of polio caused by wild-type poliovirus in the United States was in 1979; since that time, the only cases of indigenous paralytic polio reported in the United States have been associated with OPV.
Until 2000, OPV was used exclusively in the United States and around the world to control poliovirus. However, because of the risk for vaccine-associated paralytic poliomyelitis (VAPP) with OPV, an all-IPV schedule is now recommended in the United States. IPV is now the only polio vaccine available in the United States. OPV is still the only vaccine recommended in countries in which polio remains endemic.
Inactivated Poliovirus Vaccine
IPV, developed by Jonas Salk in the early 1950s, consists of formalin-inactivated noninfectious viral particles. The currently available vaccine is an enhanced formulation that contains higher concentrations of all three poliovirus serotypes and is highly immunogenic against all three. Nearly 100% of children have antibodies to all three
serotypes of poliovirus after two doses of IPV in the first year of life and a booster dose in the second year of life. Therefore, IPV is at least as immunogenic as OPV.
serotypes of poliovirus after two doses of IPV in the first year of life and a booster dose in the second year of life. Therefore, IPV is at least as immunogenic as OPV.
Because IPV is a killed virus vaccine, adverse events associated with vaccination are exceedingly rare. No risks of VAPP or of spread to immunocompromised persons are associated with IPV. Therefore, the vaccine can be administered to immunodeficient persons and their contacts. The only contraindication to IPV immunization is a severe allergic reaction to a previous dose or to streptomycin, polymyxin B, or neomycin, which the vaccine contains in trace amounts.
Oral Poliovirus Vaccine
OPV, developed by Albert Sabin and licensed for use in 1961, is a live attenuated virus vaccine containing all three poliovirus strains. The vaccine is highly immunogenic and provides durable protection that is probably life long. OPV induces mucosal immunity in the oropharynx and intestine and the virus is shed in the pharynx and feces for weeks after vaccination.
Because the virus is shed in the stool and saliva, it can be transmitted to close contacts and provide protection for those not previously immunized. OPV remains the vaccine of choice in many parts of the world and was the vaccine of choice for decades in the United States because of its:
Low production cost
Ease of administration and acceptance by patients
Ability to transmit immunity to and protect unimmunized persons (herd immunity)
Ability to induce intestinal immunity
The problem with OPV is its potential to cause paralytic disease in vaccine recipients and their contacts. The overall risk for VAPP, when both recipients and contacts are included, is 1/2.4 million doses. The risk is highest following the first dose (approximately 1/760,000 doses). The risk for VAPP is 3200- to 6800-fold higher in immunodeficient than in immunocompetent persons. Licensed OPV is no longer available in the United States.
Measles Vaccine
The current measles vaccine is a live attenuated wholevirus vaccine grown in chicken embryo cell culture. Although monovalent measles vaccine is available, it is preferable to administer measles vaccine in combination with rubella and mumps vaccines (MMR vaccine).
Immunity to measles develops in approximately 95% of children following vaccination at 12 to 15 months of age. When children are given two doses of the vaccine separated by at least 4 weeks, immunity develops in more than 99%. Because of this 5% primary vaccine failure rate with one dose of the vaccine, a two-dose schedule is routinely recommended. Measles outbreaks in the United States in the late 1980s and early 1990s were a direct result of poor immunization rates in preschool children. Since that time, the incidence of measles in the United States has fallen, and currently the incidence of measles in this country is at an all-time low. Although indigenous cases of measles are rare, imported cases from other countries continue to occur.
The seroconversion rates in infants younger than 1 year who are immunized with measles vaccine are significantly lower than those in children 1 year of age and older. This finding has been attributed to the presence of maternal passive antibody, although deficiencies in humoral immunity in the first year of life may contribute to the lack of immunogenicity of the vaccine. However, during an outbreak, the vaccine can be given as early as 6 months of age. Children immunized before their first birthday must be revaccinated at 12 to 15 months of age and then receive a third dose at least 1 month after the second dose.
Adverse Events
A fever of 39.4°C (102.9°F) or higher develops in approximately 5% to 15% of vaccinees approximately 7 to 12 days after vaccination. The seizures associated with these fevers are usually simple febrile seizures and are not associated with long-term sequelae. Transient rashes occur in 5% of vaccinees. A transient thrombocytopenia occurs rarely, and encephalitis or encephalopathy is estimated to be associated with fewer than one in 1 million doses administered. Several recent studies, as well as an Institute of Medicine Immunization Safety Review Committee report, have refuted a causal relationship between measles vaccine and autism.
The following individuals should not receive measles vaccine:
Pregnant women
Children who have had an anaphylactic reaction to a previous dose of measles vaccine
Persons with immunocompromising conditions (except asymptomatic human immunodeficiency virus [HIV] infection; see subsequent text)
In addition, in children who have recently received Ig, measles vaccination should be delayed for a specified period on the basis of the Ig preparation and dose received (see Table 2.1).
Human Immunodeficiency Virus Infection and Measles Vaccine
Infants and children infected with HIV are at risk for severe complications of measles infection, such as severe pneumonia. Therefore, measles vaccine should be given to persons with asymptomatic HIV infection and those with symptomatic infection but without severe immunosuppression on the basis of CD4= T-lymphocyte counts and percentages. Individuals infected with HIV who have severe immunosuppression
should not receive measles vaccine because of the risk for vaccine-related pneumonia.
should not receive measles vaccine because of the risk for vaccine-related pneumonia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree