Chapter 3 Imaging for Discogenic Pain
 The decision to image patients with possible discogenic pain should carefully weigh risk and cost against possible benefit.
The decision to image patients with possible discogenic pain should carefully weigh risk and cost against possible benefit. Severe nuclear signal loss (black disc) or severe loss of disc space height strongly predicts a painful disc.
Severe nuclear signal loss (black disc) or severe loss of disc space height strongly predicts a painful disc. When nuclear signal is intermediate, the inflammatory markers of the high intensity zone (HIZ) and endplate marrow change come into play.
When nuclear signal is intermediate, the inflammatory markers of the high intensity zone (HIZ) and endplate marrow change come into play. When an HIZ is seen in combination with a disc protrusion, it very strongly predicts a painful disc.
When an HIZ is seen in combination with a disc protrusion, it very strongly predicts a painful disc. Marrow endplate change of type I or type II involving greater than 25% of the vertebral body is uncommon but very strongly predicts a painful disc.
Marrow endplate change of type I or type II involving greater than 25% of the vertebral body is uncommon but very strongly predicts a painful disc. All spine imaging studies have a major specificity fault: asymptomatic degenerative phenomena on imaging studies are common, and increase with advancing age.
All spine imaging studies have a major specificity fault: asymptomatic degenerative phenomena on imaging studies are common, and increase with advancing age. Imaging carries risk: cost, radiation exposure, labeling the patient as suffering from a degenerative process, and prompting unwarranted minimally invasive and surgical interventions.
Imaging carries risk: cost, radiation exposure, labeling the patient as suffering from a degenerative process, and prompting unwarranted minimally invasive and surgical interventions.Introduction
Axial back pain, of which discogenic pain is a subset, is extremely common in Western societies. It is the most common and expensive cause of work disability in the United States.1 Recent data suggest that approximately 26% of U.S. citizens have experienced low back pain within the previous 3 months.2 The use of advanced imaging in the evaluation of back pain has dramatically increased in recent years; lumbar spine magnetic resonance imaging (MRI) scanning (measured by Medicare use) increased 307% in the 12-year interval 1994 to 2005.3 In the year 2002, the number of physician office visits related to back pain in the United States amounted to 890 million.2 This increasing use of imaging often provides little if any value to the patient. The inconsistent and often incoherent use of imaging is manifest in the large regional variations in the intensity of spine imaging across the United States; from one third to two thirds of all spine computed tomography (CT) and MRI studies are judged to be inappropriate when measured against established guidelines.3 The purpose of this chapter is to examine the existing literature regarding the imaging evaluation of the patient with suspected discogenic pain, in part with the hope that the unreasoned use of imaging may be curtailed. It is only when the literature underpinning the interpretation and significance of imaging is well understood that the clinician can effectively incorporate imaging into his or her diagnostic armamentarium.
It must also be remembered that most back pain is benign and self-limiting and benefits neither from imaging nor intervention. A generation ago, it was thought that acute low back pain was almost exclusively self limiting, with 90% of low back pain resolving within 2 months.4 More recent literature is less optimistic, but a benign course still dominates. A study by Von Korff and associates5 evaluated patients with a recent history of low back pain and found that 6 months from onset 21% of patients had no pain and 55% had mild pain with low disability. Only 14% had significant disability with moderate-to-severe limitation of function. A Dutch study6 found that, although 70% of acute patients with low back pain have persistent pain 4 weeks from onset, at 12 weeks only 35% experience persistent discomfort, and at 1 year only 10% have persistent pain. Many of these episodes of low back pain are thought to result from muscular strains, ligamentous sprains, or nonspecific degenerative phenomenon, which elude specific diagnosis in up to 85% of cases.1
In this context the primary role of imaging is to detect underlying systemic disease, which presents as low back pain. Such disease is uncommon, as detailed in the differential diagnosis of back pain compiled by Jarvik and Deyo in Table 3-1.1 In patients presenting to a primary care setting with back pain, approximately 0.7% have metastatic neoplasm as the underlying cause. Spine infections, including spondylodiscitis and epidural abscess, account for only 0.01%. Osteoporotic compression fractures are relatively common at 4%, whereas inflammatory spondyloarthropathies account for 0.3%. Characterization of neurological impairment that requires intervention is also a primary goal of imaging, typically because of disc herniation or central canal compromise. This typically presents as radicular pain, radiculopathy, or the syndrome of spinal stenosis; confusion with discogenic pain is unlikely. Although the use of imaging to identify axial pain generators is much discussed and often initiated, it rests on a far less firm foundation than its primary functions of identifying systemic disease and characterizing neurological impairment.
Table 3-1 Differential Diagnosis of Low Back Pain
| Mechanical Low Back or Leg Pain (97%) | Nonmechanical Spine Conditions (1%) | Visceral Disease (2%) |
|---|---|---|
| Lumbar strain or sprain (70%) | Neoplasia (0.7%) | Pelvic organ involvement |
| Degenerative process of disc and facets | Multiple myeloma | Prostatitis |
| (usually related to age) (10%) | Metastatic carcinoma | Endometriosis |
| Herniated disc (4%) | Lymphoma and leukemia | Chronic pelvic inflammatory disease |
| Spinal stenosis (3%) | Spinal cord tumors | Renal involvement |
| Osteoporotic compression fracture (4%) | Retroperitoneal tumors | Nephrolithiasis |
| Spondylolisthesis (2%) | Primary vertebral tumors | Pyelonephritis |
| Traumatic fractures (<1%) | Infection (0.01%) | Perinephric abscess |
| Congenital disease (<1%) | Osteomyelitis | Aortic aneurysm |
| Severe kyphosis | Septic discitis | Gastrointestinal involvement |
| Severe scoliosis | Paraspinous abscess | Pancreatitis |
| Transitional vertebrae | Epidural abscess | Cholecystitis |
| Spondylolysis | Shingles | Penetrating ulcer |
| Internal disc disruption or discogenic back pain | Inflammatory arthritis | |
| Presumed instability | (often HLA-B27 associated)(0.3%) | |
| Ankylosing spondylitis | ||
| Psoriatic spondylitis | ||
| Reiter syndrome | ||
| Inflammatory bowel disease | ||
| Scheuermann disease (osteochondrosis) | ||
| Paget disease |
From Jarvik JG, Deyo RA: Diagnostic evaluation of low back pain with emphasis on imaging, Ann Intern Med 137(7), 2002.
Basic Imaging Principles
It is well established that there is no role for imaging in the patient who presents with acute back and/or leg pain in the absence of signs of systemic disease or neurological impairment that may require intervention. Chou and associates7 performed a meta-analysis of all randomized controlled trials comparing immediate imaging (radiographs, CT, MRI) vs. clinically directed care in the acute back pain patient. There were six qualifying trials; the analysis showed no significant differences in pain or function in imaged vs. nonimaged patients in either the short term (3 months) or long term (6 to 12 months). They concluded that “lumbar imaging for low back pain without indications of serious underlying conditions does not improve clinical outcomes”.7 In addition to being ineffective, early use of imaging is costly; a cost-effectiveness analysis performed by Liang and Komaroff8 demonstrated that simply performing radiographs at the initial presentation of back pain results in a cost of $2000 (1982 dollars) to alleviate a single day of pain. The lack of utility of imaging in the acute setting was also well illustrated by a 5-year prospective observational study performed by Carragee and colleagues.9 A large cohort of asymptomatic persons who were at risk for developing back pain as a result of physically intensive vocations underwent MRI scanning. This cohort was followed periodically over 5 years; a subset ultimately presented to a physician with back or leg pain, at which time a second MRI was performed. Less than 5% of the MRI scans obtained at the time of acute presentation with back or leg pain showed clinically relevant new findings; virtually all of the “abnormalities” noted on the scans obtained at presentation with back pain had been present on imaging obtained when the patient was asymptomatic. Only direct evidence of neural compression in patients with a corresponding radicular pain syndrome was assessed to be useful imaging information. Psychosocial factors, not the morphology seen by imaging, were the primary predictors of the degree of disability caused by back pain.9
Analysis of such data has resulted in recommendations against the use of imaging in the patient who presents with acute back pain. The imaging recommendations of the American College of Radiology were recently restated by Bradley.10 Imaging in the patient who presents with acute low back pain is not indicated except in the presence of “red flag” features, which include recent significant trauma, minor trauma in a patient older than 50, weight loss, fever, immunosuppression, history of neoplasm, steroid use or osteoporosis, age greater than 70, known intravenous drug abuse, or a progressive neurological deficit with intractable symptoms. Similarly, a joint recommendation from the American College of Physicians and the American Pain Society in 2007 stated that imaging should not be obtained in patients with nonspecific low back pain.11 Imaging should only be performed when severe or progressive neurological deficits are present or when serious underlying systemic disease is suspected. Furthermore, patients with signs or symptoms of radiculopathy or spinal stenosis should be imaged only if they are candidates for surgery or epidural steroid injection. These recommendations emphasize the primary role of imaging as a means of detecting underlying systemic disease, typically neoplasm, infection, or unsuspected traumatic injury.
Risks and Benefits of Imaging for Back Pain
However, there are risks associated with imaging, which include the labeling effect, radiation exposure, cost, and provocation of intervention. The labeling effect refers to patient self-identification as suffering from a degenerative process. Degenerative phenomena are inevitably identified on any imaging study, as we discuss further regarding specificity of imaging. Unless appropriately educated to the contrary, patients may perceive this as representing the start of an inevitable downward spiral of spine degeneration. This may lead to fear-avoidance behaviors with diminished activity, deconditioning, and depression. A recent Cochrane database review established the effectiveness of active patient education, particularly in the setting of acute low back pain.12 The irrelevance of degenerative findings on imaging studies and the importance of maintaining core muscle strength and high activity levels must be reinforced at every patient encounter.
Exposure to radiation from radiographs or CT generates a cumulative risk. Effective absorbed radiation dose is measured by the sievert (Sv); the average annual natural background exposure in North America is approximately 3 mSv.13 A frontal and lateral chest radiograph may be considered the common currency of radiation exposure; it incurs a dose of 0.1 mSv.13 A three-view lumbar spine radiographic series is worth 1.5 mSv, or 15 chest radiographic series.13 A dose of 6 mSv is typical for a lumbar spine CT scan (60 chest radiographs). A technetium bone scan has a similar dose of 6.3 mSv.13 For context, an abdomen and pelvis CT study incurs 14 mSv.13 All radiation exposure cumulates over the patient’s lifetime and contributes to a risk of radiation-induced neoplasm. Radiograph-based imaging studies must be used with careful consideration of risk and anticipated benefit.
Imaging is costly. In the United States the medical imaging community incurs more than $100 billion of societal cost per year. The 2009 Medicare reimbursements for lumbar spine imaging studies were radiographs: $41; noncontrast CT: $264; myelogram: $506; noncontrast MRI: $439; whole body positron emission tomography (PET)/CT: $1183; bone scan with single photon emission computed tomography (SPECT): $261.14 Nominal fees are typically three to five times the Medicare reimbursements. It is easy to appreciate how quickly imaging costs can accrue.
Finally, and perhaps most important, imaging the spine increases the likelihood that there will be minimally invasive or surgical intervention. Jarvik and associates15 showed that early MRI imaging leads to more surgical interventions despite equivalent pain and disability profiles when compared with nonimaged patients. Similarly, Lurie, Birkmeyer, and Weinstein16 noted that the large regional variations (twelvefold) in surgical rates for spinal stenosis can be directly explained by the frequency of CT and MRI use. When we image, we intervene. This is particularly significant in the realm of discogenic pain, in which we have no well-validated therapies, whether surgical or minimally invasive. A recent meta-analysis of surgical fusion for axial back pain noted that three of the four randomized controlled trials in the literature showed no benefit for fusion when compared with structured conservative care.17 The only randomized study showing minimal benefit for fusion had no organized conservative care as the control arm. Similarly, none of the numerous device-based image-guided interventions that have entered (and left) the marketplace in the United States in the last two decades have shown substantial benefit in randomized trials. Most of the procedures have never even undergone rigorous evaluation with a randomized controlled trial. In a highly imperfect medical marketplace, imaging frequently leads to interventions that have little or no evidence of efficacy, only demonstrable risk and cost.
Sensitivity and Specificity of Spine Imaging
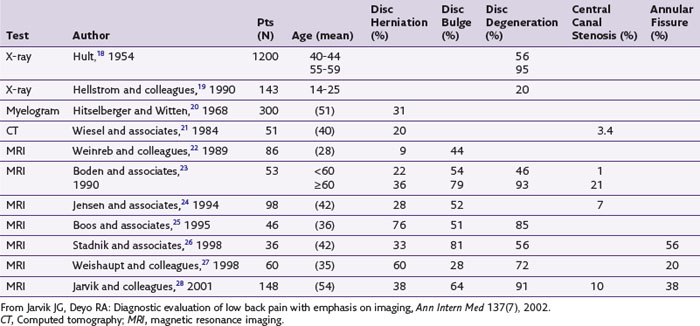
Having weighed the risks and benefits of imaging and chosen to proceed, it is imperative to understand the sensitivity and specificity shortcomings that are common to all spine imaging modalities. We first consider specificity. Imaging findings of degenerative phenomenon in both the anterior and posterior columns of the spine are commonly seen in asymptomatic populations and cannot be considered causal of an individual patient’s pain syndrome. In adult populations without low back pain, the prevalence of degenerative changes is significant, with 56% to 72% of asymptomatic adults showing evidence of disc degeneration, 20% to 81% demonstrating disc bulges, 27% to 33% having disc protrusions, 0% to 18% demonstrating disc extrusions, 6% to 56% having annular fissures or high intensity zones, and 2% to 7% demonstrating endplate marrow changes (Table 3-2).1
More recently studies have addressed the prevalence of degenerative findings in younger populations, primarily in Scandinavian countries, using MRI. These were population-based studies without respect to symptomatology. Kjaer and associates,29 studying children age 13, found a 21% prevalence of disc degeneration. In a study of adolescents, Salminen and colleagues30 found a 31% prevalence of disc degeneration in 15-year-olds, which rose to 42% in 18-year-olds. Takatalo and associates31 evaluated 558 young adults ages 20 to 22.31 Using the five-point Pfirrmann classification of disc degeneration, they noted disc degeneration of grade 3 or higher in 47% of these young adults. There was a higher prevalence in males (54%) than in females (42%). Multilevel degeneration was identified in 17%.
There is also a basic sensitivity fault present in all spine imaging modalities. This applies most significantly to neuroclaudicatory processes in which compression of neural structures results in radicular pain or neural dysfunction. It is well demonstrated that pain caused by neural compressive processes is exacerbated by extension positioning and axial load. The cross-sectional area of the lumbar central canal, lateral recesses, and foramina are known to diminish with extension and axial load.32 Advanced imaging performed in a supine, psoas relaxed position may be insensitive to dynamic neural compressive lesions. This can be overcome by imaging with axial loading devices in conventional CT or MRI scanners,33 or by imaging with MRI scanners that allow standing or seated positions.34 The imaging trade-off is that such MRI scanners are of low field strength and produce limited image quality.
Imaging Findings Predictive of Discogenic Pain
The changes seen in the intervertebral disc with aging in the asymptomatic person and in the patient with low back pain are of multifactorial origin. Mechanical, traumatic, nutritional, and genetic factors are involved. For purposes of this discussion, the term degenerative encompasses both normal aging and the similar but more rapidly progressive pathological process. Such phenomena are ubiquitous, with 85% to 95% of adults over the age of 50 showing evidence of degenerative disc changes at autopsy.4 The consensus terminology agreed on by multiple spine imaging medical and surgical societies uses the term spondylosis deformans, originally introduced by Schmorl and Junghanns, to describe normal aging phenomenon; this primarily reflects changes in the annulus fibrosis and adjacent vertebral apophyses resulting in anterior and lateral endplate osteophytes.35 Such osteophytes are present in 100% of skeletons of individuals over 40 years of age.36 Intervertebral osteochondrosis is the term used to describe pathological (although not necessarily symptomatic) degeneration; it involves failure of the nucleus pulposus to effectively disperse axial load with concurrent changes in the vertebral endplates and extensive fissuring in the annulus fibrosis.35 Pathological changes in this process include posterior vertebral osteophytes, endplate erosions, extensive annular fissuring, and reactive bone marrow changes.
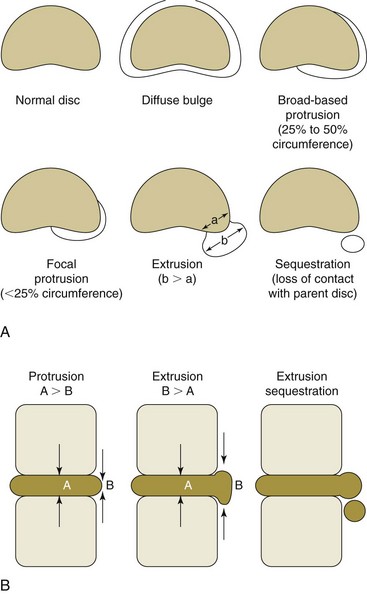
When the disc-endplate complex undergoes degeneration with annular failure, disc contour alteration (i.e., herniation) may occur. Discussion of disc herniation requires a brief commentary on nomenclature. Description of disc herniation has historically been chaotic, with no common terminology among various medical and surgical specialties. This was resolved by a combined task force of the North American Spine Society (NASS), American Society of Spine Radiology (ASSR), and the American Society of Neuroradiology (ASNR), whose recommendations were published in 2001.35 This imaging lexicon should allow us to communicate better across specialty and regional lines; it is depicted in Fig. 3-1. Herniation is the broad term describing displacement of disc material beyond its normal intervertebral disc space. If the extent of the herniation is greater than 50% of the circumference of the disc, it may be considered a bulge. A localized herniation is defined as involving less than 50% of the disc circumference. Localized herniations may be further divided into broad-based herniations, which encompass 25% to 50% of the disc circumference, and focal herniations, which constitute less than 25% of the circumference. The distinction between protrusion and extrusion is one of shape. In a protrusion the width of displaced disc material in any plane does not exceed the width of its base or the aperture through which the disc material had left its normal position. In an extrusion the width of the displaced disc material exceeds its base or aperture in any plane. The presence of an extrusion shape suggests that there has been complete disruption of the outer annulus and disc material has entered the epidural space. Sequestration is the term for loss of continuity of a disc fragment with the parent disc from which it arose. Displacement of disc material away from the parent disc is termed migration.
The existing literature that seeks to identify imaging findings predictive of discogenic pain deals primarily with MRI findings, which is the focus of our discussion. The findings to be evaluated include (1) loss of disc space height, (2) alterations of disc contour, (3) generalized alterations in T2 signal within the disc, (4) endplate marrow changes, and (5) the presence of HIZs or fissures within the posterior disc annulus. These imaging features are examined initially as independent variables with subsequent discussion of the more limited literature in which they are combined in a multivariate analysis. A significant portion of the presented data was drawn from a systematic review of imaging and clinical markers of axial pain generators in the lumbar spine performed by Hancock and colleagues.37 Additional studies not included in that report or published subsequent to it have been added. A common set of measures was compiled from the numerous studies: sensitivity, specificity, positive predictive value, negative predictive value, and likelihood ratios. When imaging features were quantified (e.g., T2 signal loss in the disc was reported as normal, moderate, or severe), a threshold was used. Original data were combined and recalculated to reflect setting a detection threshold as moderate (including moderate and severe cases) or severe only. Since the diagnosis of discogenic pain may provoke therapeutic interventions (most of which carry risk and have unproven efficacy), emphasis is placed on those measurements that inform us about false-positive results: specificity (true negatives/true negatives + false positives) and positive predictive value (true positives/true positives + false positives). These measures can be conflicting when class sizes vary and depend on the prevalence of the condition being studied.38 Likelihood ratios (LRs) are prevalence independent, given by sensitivity/(1 − specificity). The higher the positive LRs (+LR), the more likely it is that a patient with a positive test does have the disease, here discogenic pain. When the +LR exceeds 2, the finding is considered useful when the confidence interval does not cross 1. The lower a negative likelihood ratio (−LR), the more likely a patient with a negative test does not have the disease. When the −LR is less than 0.5, the finding is considered informative when the confidence interval does not include 1.
Before proceeding with examination of individual imaging findings, we must first consider the gold standard dilemma. There is no surgical or pathological marker of a painful intervertebral disc. The most restrictive current standard for a painful disc is a concordant response to manometrically controlled provocation discography with nonpainful control levels as defined by the practice guidelines of the International Spine Intervention Society (ISIS). 39 Discography is discussed in more detail elsewhere in this volume. The target audience of this volume likely advocates for, or is at least accepting of, the use of discography. However, it must be remembered that examination of the same body of evidence by different physician societies regarding the validity of discography has resulted in diametrically opposed recommendations regarding its use. The ISIS,39 the North American Spine Society (NASS),40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree