Key Clinical Questions
What key clinical entities must be considered in the initial assessment of a hospitalized patient with hypoxemia?
What are initial diagnostic tests and assessments that should be obtained in the hypoxemic patient?
What are the potential pitfalls of reliance on pulse oximetry to define hypoxemia, and how are these avoided?
How should supplemental oxygen be delivered in the hypoxemic hospitalized patient?
A 63-year-old white female with a history of chronic obstructive pulmonary disease (COPD), coronary artery disease with three stents, hypertension, obesity, diabetes mellitus (DM), and lung cancer was admitted to the hospital from clinic with worsening shortness of breath over the last 2 months, requiring oxygen supplementation. She had been on 2 liters per minute (LPM) of oxygen via nasal cannula for the past 3 years for COPD, but in the past few months has experienced worsening shortness of breath and dyspnea on exertion. At this point she cannot walk to the bathroom in her house without getting short of breath. She admited to orthopnea, lower extremity edema, fatigue, and chest pain as well. She now required 4–8 LPM of oxygen in order to maintain an oxygen saturation > 89%. Her COPD was diagnosed in 2005, and she has been on supplemental oxygen since then. She had lung cancer in 1996, which was treated with lobectomy and radiation therapy. She had a 50 pack-year tobacco history but quit smoking 12 years ago. Pertinent medications included hydrochlorothiazide, simvastatin, pioglitazone, fl uticasone/salmeterol inhaler, and tiotropium inhaler. Her physical exam was notable for an oxygen saturation of 94% on 4 liters, but she desaturated to 81% when semirecumbent. Crackles were heard in the left lung base, and she became quite dyspneic and tachypneic upon minimal effort. A 1/6 systolic ejection murmur was heard, along with a loud P2 and paradoxical splitting of S2. Lower extremities had +1 pitting edema, and she had mild digital clubbing What were the next steps needed to appropriately evaluate and treat this patient’s hypoxia? |
Introduction
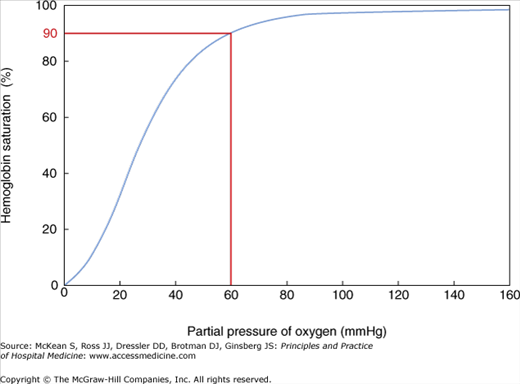
Hypoxia is defined by an abnormally low arterial oxygen tension. A PaO2 of 60 mm Hg generally corresponds with the point on the oxygen–hemoglobin dissociation curve in which hemoglobin is 90% saturated. The curve is steep at this point, and further decreases in oxygen tension correspond with dramatic falls in hemoglobin saturation and resultant inadequate oxygen delivery to tissues (Figure 93-1). Oxygen affinity can be affected by pH, carbon dioxide (CO2), 2,3-diphosphoglycerate (2,3-DPG), and temperature. As pH decreases and CO2 increases, oxygen is more readily released, shifting the oxyhemoglobin curve to the right, increasing delivery of oxygen to the tissues. Red blood cells contain 2,3-DPG, which helps modulate oxygen affinity. Increasing levels of 2,3-DPG decrease the oxygen affinity, also shifting the dissociation curve to the right. Elevated body temperature shifts the dissociation curve to the right, helping to unload oxygen at a time when additional oxygen to tissues may be needed.
Hypoxia is a common and an important cause of mortality and morbidity in the hospital. Therefore, rapid evaluation and treatment is critical to avoid serious complications resulting from hypoxia. History and physical exam alone is not suffi cient to rapidly detect hypoxia, and other measurements should be used to accurately and effi ciently detect hypoxia, including pulse oximetry and blood gas analysis. This chapter will cover the etiology and pathophysiology of hypoxia, considerations for diagnosis of hypoxia, common pitfalls, and treatment options to correct hypoxia.
Pathophysiology and Mechanisms of Hypoxia
There are five general mechanisms that cause hypoxia: ventilation/perfusion (V/Q) mismatch, right to left shunt, hypoventilation, diffusion abnormalities, and reduced inspired oxygen tension (Table 93-1). Only the first four of these are clinically relevant, and only the first three generally cause hypoxia at rest. It is important for the clinician at the bedside to be familiar with these mechanisms and common disease states responsible for them. This will allow for more accurate diagnoses and will facilitate appropriate therapy. The underlying cause of hypoxia can often be elucidated with simple tests such as arterial blood gas (ABG) assessment and chest radiographs. Additionally, knowing how much the PaO2 increases with oxygen supplementation can help differentiate shunt from other causes of hypoxia.
| Mechanism | Example | Diagnostic Clues |
|---|---|---|
| Hypoventilation |
|
|
| Ventilation/perfusion (V/Q) mismatch |
|
|
| Right to left shunt |
|
|
| Diffusion impairment |
|
|
| Reduced inspired oxygen tension |
|
|
The most common cause of hypoxia is V/Q mismatch. Areas of the lung with low ratios of ventilation to perfusion result in low alveolar oxygen (O2) tension. In most situations of V/Q mismatch, there are areas with low V/Q ratios, normal V/Q ratios, and high V/Q ratios. While areas of high V/Q ratio will have a higher alveolar oxygen tension, this will not offset the low PaO2 from low V/Q areas. This is because the hemoglobin saturation does not change very much from normal V/Q areas to high V/Q areas; therefore the increase in arterial oxygen content from these areas is small and does not offset the decrement in oxygen content from the low V/Q areas.
Common causes of V/Q mismatch include obstructive lung diseases such as asthma and chronic obstructive pulmonary disease (COPD), but V/Q mismatch is seen in many other lung diseases, including pulmonary embolism, pulmonary vascular diseases, and interstitial lung diseases. In a pulmonary embolism, gas exchange abnormalities occur from increased alveolar dead space, but hypoxia can also occur due to V/Q mismatch, right to left shunting, and a low mixed venous O2 level. Low V/Q ratios develop because blood flow is redistributed away from the obstructed vessel, resulting in overperfusion of normal lung regions. Humoral mediators released by platelets stimulate bronchoconstriction, causing atelectasis, which complicates hypoxia. In addition, physiologic shunting occurs because of increased flow through some areas of low V/Q ratios. V/Q mismatch is characterized by an increased alveolar-arterial (A-a) gradient. The A-a gradient is the difference in PaO2 between the alveoli and arterial blood (see later discussion for additional information).
Hypoxia caused by V/Q mismatch is improved by relatively low levels of supplemental oxygen, which helps differentiate V/Q mismatch from hypoxia caused by a shunt (see later discussion), as a shunt is not readily corrected by increasing levels of FiO2. Even with supplemental oxygen, shunted blood does not come into contact with alveoli with a higher PO2. However, with supplemental oxygen in V/Q mismatch, in the low V/Q regions, the PaO2 increases with a higher FiO2, and the blood flow to these regions will have a higher capillary oxygen content, therefore producing a higher PaO2.
Right to left shunt refers to conditions in which deoxygenated blood from the right side of the heart bypasses oxygenation in the lungs and goes to the left side of the heart. One way to consider shunt is in terms of V/Q ratios. The extreme case of V/Q ratio in which there is perfusion but no ventilation is synonymous with shunt. Shunt can occur due to anatomic abnormalities such as intracardiac shunts or arteriovenous malformations. Shunt also occurs due to alveolar filling processes such as pneumonia, acute respiratory distress syndrome, or alveolar collapse (ie, atelectasis). As already noted, shunt is less responsive to supplemental oxygen than other causes of hypoxia. This can be helpful in diagnosing shunt.
In hypoventilation, alveolar CO2 increases; as a result alveolar O2 must decrease. Alveolar hypoventilation is a common occurrence in hospitalized patients. It can be caused by narcotic analgesics and other central nervous system depressants. There are also central hypoventilation syndromes such as obesity-hypoventilation syndrome. Conditions that cause weakness of the respiratory muscles such as Guillain-Barré syndrome, amyotrophic lateral sclerosis, and myasthenia gravis may result in hypoventilation, as will disorders of the chest wall such as kyphoscoliosis. An ABG taken in the setting of hypoventilation will reveal an elevated CO2 but will have a normal A-a gradient. The latter may be increased if hypoventilation is coupled with atelectasis, as it frequently is. Furthermore, hypoxemia due to hypoventilation is readily corrected by an increase in fraction of inspired oxygen (FiO2). However, one should use caution in patients with hypoxia due to hypoventilation. It is critical to treat the hypoxia, but patients with hypoventilation syndromes may have a blunted respiratory response to hypercarbia and may depend on their hypoxic respiratory drive. Their hypoventilation and CO2 retention may worsen in response to supplemental oxygen, and CO2 narcosis can ensue. Additionally, increasing PaO2 without improving hypoventilation will mask the ability to detect ongoing hypoventilation with pulse oximetry. In other words, hypoventilation and hypercarbic respiratory failure may continue and worsen, but oxygen saturations will remain above 90%. Ventilatory assistance, frequently with noninvasive ventilation, is often necessary to treat hypoxia due to hypoventilation.
Lung diseases that impair diffusion of oxygen across the alveolar capillaries and into the bloodstream can result in hypoxia. However, at rest, gas exchange is completed by the time blood has moved a third of the way through the alveolar capillaries. Therefore, even in the setting of severe decrements in diffusing capacity, there is usually enough time in the alveolar capillaries for gas exchange to be completed, and hypoxia does not occur. However, exercise and other conditions that decrease the transit time of blood in alveolar capillaries will lead to hypoxia in the setting of impaired diffusion. Common conditions that cause impaired diffusion are disorders of interstitial inflammation such as idiopathic pulmonary fibrosis or pneumocystis pneumonia. Diseases that result in loss of alveolar surface area, such as emphysema, will also lead to impaired diffusion. Furthermore, these diseases may also be associated with V/Q mismatch and have mixed etiologies for hypoxia. Hypoxia from impaired diffusion is often associated with desaturation with activity and corrects relatively easily with supplemental oxygen.
Hypoxia will occur if the inspired oxygen tension is reduced. Fortunately this does not occur in hospitals but is an important phenomenon in mountaineers. Compensatory mechanisms cause hyperventilation and the ABG value shows a low PaCO2 and a low PaO2. This condition also improves with supplemental oxygen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree