Many non-anesthetic pharmaceuticals have the potential to cause hypoglycemia in susceptible individuals; many more have drug–drug interactions that may potentiate the action of oral hypoglycemic agents and indirectly cause hypoglycemia. For example, many medications compete for plasma protein binding sites, displacing sulfonylurea medications and increasing the free fraction within the plasma, while others inhibit metabolism by competing for hepatic enzymes responsible for metabolism. Some of these medications are often initiated around the time of surgery. Fortunately, the hypoglycemic effect of most medications initiated perioperatively is weak and resultant hypoglycemia rarely clinically encountered.[12,13]
Postoperative hypoadrenalism may feature hypoglycemia along with the other clinical features, usually including hypotension, hypothermia, confusion, myalgias, and diarrhea. Hypoadrenalism may have a pharmacological cause for patients on chronic exogenous steroid therapy with suppression of pituitary–adrenal axis or patients receiving etomidate for induction of general anesthesia. The incidence of hypoadrenalism among surgical patients whose chronic exogenous steroids are abruptly interrupted appears to be about 1% to 2%.[14,15] Adrenalectomy may produce hypoadrenalism, as may other adrenal procedures. Hypoadrenalism has very rarely been observed after resection of pheochromocytoma.[16] Hypoadrenalism may also be a part of panhypopituitarism, seen most frequently after procedures near the sella turcica or after sustained hypotension from hemorrhage causing pituitary apoplexy (Sheehan’s syndrome).
Postoperative hypoglycemia may be caused by gastric dumping syndrome, frequently observed after esophageal, gastric, or bariatric surgery. Gastric dumping syndrome may cause hypoglycemia when large food particles are prematurely delivered to the small bowel, resulting in poor glucose absorption and a relative excess of endogenous insulin. Although clinically important, this typically presents after feeding has resumed and relatively late in the postoperative course.[17]
Technical limitations of glucose meters also contribute to hypoglycemia. Most meter errors are due to operator errors or calibration errors, but meter design may influence performance under clinical conditions. Acetaminophen, dopamine, and mannitol, all of which may be administered perioperatively, interfere with the performance of meters which employ a peroxide reduction detection method for glucose measurements, leading to pseudohypoglycemia, while meters using an amperometric technique are unaffected by these medications.[18] Hypotension may also lead to pseudohypoglycemia as poor perfusion of the extremities allows for greater utilization of glucose and lower measured levels. This pseudohypoglycemia is not a failure of the meter to measure the sample accurately, but rather a discrepancy between capillary and venous samples. Glucose meters utilizing a glucose–oxygen-based measurement may be affected by blood oxygenation. Supplemental oxygen can cause false depressed glucose readings and hypoxia can cause falsely elevated measurements. Severe acidosis (pH <6.95) may also lead to spuriously low glucose readings with many types of meters.[18]
A root cause analysis of severe hypoglycemia (40 mg/dl) recognized during surgery among 80,379 patients at a large tertiary care hospital identified preventable errors in 47% of cases.[19] Ineffective communication (such as providers being unaware that the patient received/administered insulin that morning), circulatory shock, failure to monitor, and excessive insulin administration were the four most commonly identified contributing factors. This serves to underscore the importance of communication among the various providers of different disciplines in the postoperative period in order to avoid preventable hypoglycemic episodes due to miscommunication or failure of vigilance. In one tragic case of inadequate communication, there was a failure to report the use of icodextrin peritoneal dialysate in the postoperative period. Icodextrin falsely elevates glucose readings on some glucose dehydrogenase-based meters and contributed to excessive insulin therapy in an attempt to treat factious hyperglycemia, resulting in death.[20]
Prevention
Prevention of hypoglycemia is preferred over detection and treatment. It requires that patients at risk for hypoglycemia be identified prior to or upon admission to the hospital. For these patients, glucose monitoring should start at admission and continue in the intraoperative period. The Society for Ambulatory Anesthesia recommends a monitoring frequency of every one to two hours.[9] Considering not only the current glucose measurement, but also the rate of change will allow you to anticipate an impending hypoglycemic event and make proactive adjustments in therapy (e.g. reduction of intravenous [IV] insulin infusion or administration of an IV bolus/infusion of dextrose) to avoid it. Recognition of other precipitating factors such as discontinuation of dextrose-containing infusions, recent administration of exogenous insulin, or the presence of predisposing co-morbidities is also helpful in prevention. If a hypoglycemic event does occur, it must be treated promptly with appropriate follow-up. Care should be taken to avoid overtreatment to avoid hyperglycemia. Treatment protocols should be established and disseminated. Adequate oversight can increase adherence to, and identify deficiencies in, established hypoglycemia treatment protocols.
With proper staff education, and patient monitoring and care, hypoglycemia can be avoided. Educated staff should be able to quickly identify at-risk patients and, coupled with proper monitoring, recognize and treat a patient before clinically significant hypoglycemia develops.
Management
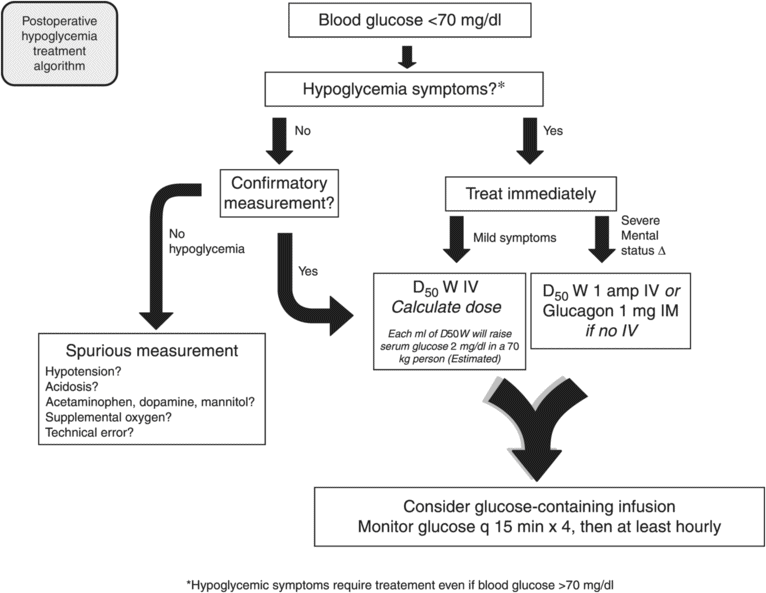
Treatment of hypoglycemia in the immediate postoperatively period is unique (see Figure 16.2). Here, it is common for the patient to be unresponsive and have IV access. Whereas treatment with oral glucose is often the first recommendation if it can be tolerated, administration of an IV bolus or infusion of dextrose should be considered the first option in the PACU.
To avoid mistreatment, aberrant glucose measurements should be scrutinized. Blood obtained from a catheter, especially a central venous catheter, is susceptible to pre-analytical error due to contamination with dextrose-containing solutions or dilution with dextrose-free solutions.[21] In addition, poor perfusion, interfering substances, and hematocrit abnormalities have been known to produce erroneous glucose readings when using portable glucose meters.[22–24] When a critical glucose value does not appear to be physiologically possible, a new sample should be drawn and/or repeat testing should be performed to confirm results.
Whether administered via an IV bolus or taken by mouth as simple or complex carbohydrates, the default amount of glucose to deliver in response to hypoglycemia is 15g. However, to avoid overtreatment, this amount should be calculated. If the body is taken as a single pool for the distribution of glucose, a simple calculation provides the estimated amount of glucose to deliver to regain euglycemia. First, the distribution volume (Vd) [in dl/kg] is estimated to be between 1.0 and 3.0.[25–27] Given the patient’s weight (Wt) [in kg], desired glucose concentration (Gdesired) [in mg/dl], and current glucose concentration (Gactual) [in mg/dl], the amount of dextrose to administer [in g] as an IV bolus is:
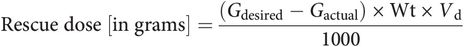
Using the glucose distribution volume of 2 dl/kg, for a 70 kg patient with a measured glucose concentration of 50 mg/dl, the appropriate rescue dose is 7 g to achieve a desired glucose value of 100 mg/dl. It is recommended that this dose is delivered as a bolus and, if no contraindication for IV fluids exists, a dextrose infusion should also be started until stable euglycemia is established. Moreover, more frequent monitoring (q 15 min) is recommended for at least one hour following the IV bolus. Here, the time taken for complete distribution of the glucose bolus from the vascular space to the interstitial space and intracellular space can be at least one hour for glycemic response to IV glucose tolerance tests, so glucose measurements following an IV bolus should be evaluated accordingly. While each ml of D50W, dextrose 50% in water raises serum glucose by approximately 2 mg/dl in the average 70 kg adult, wide variation in clinical effect of D50W has been observed in euglycemic volunteers, necessitating clinical evaluation of response to therapy and vigilant post-treatment monitoring.[28]
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree