Hypertrophic Cardiomyopathy
Robert J. Siegel
Huai Luo
Definition
Primary hypertrophic cardiomyopathy (HCM) is an inherited genetic condition in which there is left ventricular hypertrophy (LVH) that occurs in the absence of left ventricular (LV) pressure overload such as valvular aortic stenosis or systemic hypertension or hormonal stimulation from growth hormone or elevated catecholamines.
Based on early M-mode echocardiography (echo) studies, HCM traditionally has been considered to have asymmetric septal hypertrophy (ASH). However, a recent magnetic resonance imaging (MRI) study of 333 HCM patients found that concentric LVH (LVH in greater than or equal to eight segments) occurs in 54% of patients.
In addition to ASH and concentric LVH, any LV segment may be abnormally thickened.
LV apical hypertrophy (also known as Yamaguchi disease) is also another HCM variant. HCM typically has LVH with wall thickening ≥15 mm. However, genotype studies have shown that any range of wall thickness (even normal) can be associated with an HCM mutant gene.
When the LV wall is 13 to 14 mm in thickness, it is considered mild LVH in the setting of HCM. In HCM patients with a wall thickness >17 mm, the LVH is considered severe.
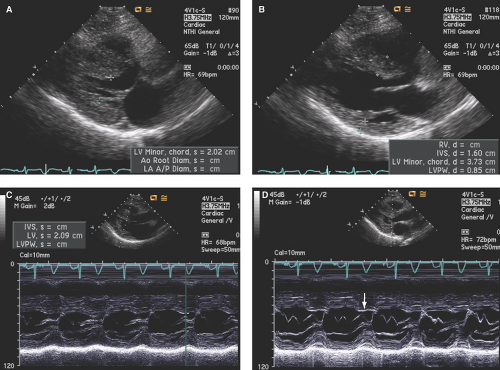
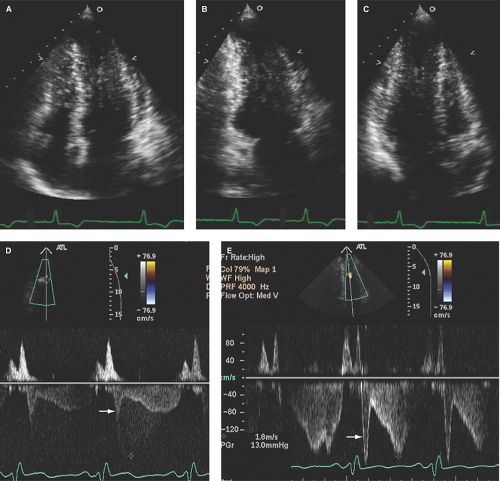
As shown in Figure 15.1A–D, in addition to LVH, the LV is generally nondilated with normal to hyperdynamic contractility. LV cavity obliteration during systole is not uncommon.
Left ventricular outflow tract (LVOT) gradients are commonly associated with HCM and usually associated with systolic anterior motion (SAM) of the mitral valve (Fig. 15.1D). However, most HCM patients do not demonstrate LVOT gradients (“obstruction”) at rest. It has been estimated that about 25% of HCM patients have resting LVOT gradients, with as many as 50% of patients developing LVOT gradients with provocations.
By the American College of Cardiology (ACC)/European Society of Cardiology consensus document, gradients are considered nonobstructive if <30 mm Hg, provokable if they rise to ≥30 mm Hg with provocation, and obstructive if the resting gradient is ≥30 mm Hg (or 2.7 m/second by Doppler).
LVOT gradient may be induced by Valsalva maneuver, preload reduction with hypovolemia or nitrates, factors that increase contractility from adrenergic stimulation from stress, or pharmacologic stimuli such as dobutamine.
It should be noted, however, that many patients with systemic hypertension and LVH also have inducible LVOT gradients. Thus, provocation of an LVOT gradient is a nonspecific finding and is not diagnostic for HCM.
Common Etiologies and Prevalence
This genetic condition occurs in approximately 1 in 500 people.
It has an autosomal dominant transmission due to mutations most often occurring in the genes coding for sarcomeric proteins. At present, 13 genes and >500 mutations have been identified.
Most patients manifest LVH during or shortly after the musculoskeletal growth phase associated with puberty and more generally by the early twenties. However, a few genetic variants may demonstrate LVH later in life. In addition, there are a group of patients with “hypertensive hypertrophic cardiomyopathy.” This entity is more commonly seen in women and the elderly.
Echocardiography
Class I or Appropriate Indications for Echocardiography
There are several American Heart Association (AHA)/ACC/American Society of Echocardiography (ASE) echo guidelines that apply to HCM patients, including delineation of Doppler echo capabilities in the adult patient with HCM (Table 15.1) and evaluation of
patients with murmur, native valve regurgitation, dyspnea, edema and/or known cardiomyopathy, or more specifically for evaluation of patients with HCM (Table 15.2).
Table 15.1 Echocardiography capabilities in the adult patient with hypertrophic cardiomyopathy
Echocardiographic Modality
M-Mode
2D
Spectral Doppler
Color Doppler
TEE
Anatomy/Pathology
Chamber size
++++
++++
–
–
++
Thickness of walls
++++
+++
–
–
+++
Relation of chambers
+
++++
–
–
+++
Systolic anterior motion of MV
++++
+++
–
–
+++
LV mass (g)
++++
++++
–
–
–
Anatomic valvular pathology
++
++++
–
–
++++
Pericardial effusion
++
++++
–
–
++
Function
Global LV systolic function (EF)
++
++++
++
–
+++
Regional wall motion
+
+++
–
–
++++
Severity of LVOT gradient
+
++
++++
+++
++
Severity of valve regurgitation
+
+
+++
+++
+++
RV and PA systolic pressure
–
–
++++
–
–
LV filling pressure
–
–
++
–
–
Stroke volume and cardiac output
+
++
+++
–
–
LV diastolic function
+
+
+++
–
–
2D, two-dimensional; TEE, transesophageal echo; MV, mitral valve; LV, left ventricle; EF, ejection fraction; LVOT, left ventricular outflow tract; RV, right ventricle; PA, pulmonary artery.
Table 15.2 Class I or appropriate indications for echocardiography in patients with suspected or known hypertrophic cardiomyopathy
In Patients with Heart Murmurs (Common Finding in Patients with HCM)
- A murmur in a patient with cardiorespiratory symptoms.
- A murmur in an asymptomatic patient if the clinical features indicate at least a moderate probability that the murmur is reflective of structural heart disease.
In Patients with Native Valvular Regurgitation (Mitral Regurgitation in HCM)
- Diagnosis; assessment of hemodynamic severity.
- Initial assessment and re-evaluation (when indicated) of LV and RV size, function, and/or hemodynamics.
- Re-evaluation of patients with mild to moderate valvular regurgitation with changing symptoms.
- Re-evaluation of asymptomatic patients with severe regurgitation.
- Assessment of changes in hemodynamic severity and ventricular compensation in patients with known valvular regurgitation during pregnancy.
- Re-evaluation of patients with mild to moderate regurgitation with ventricular dilation without clinical symptoms.
- Assessment of the effects of medical therapy on the severity of regurgitation and ventricular compensation and function.
In Patients with Dyspnea, Edema, or Cardiomyopathy
- Assessment of LV size and function in patients with suspected cardiomyopathy or clinical diagnosis of heart failure.
- Edema with clinical signs of elevated central venous pressure when a potential cardiac etiology is suspected or when central venous pressure cannot be estimated with confidence and clinical suspicion of heart disease is high.
- Dyspnea with clinical signs of heart disease.
- Patients with unexplained hypotension, especially in the intensive care unit.
- Patients exposed to cardiotoxic agents, to determine the advisability of additional or increased dosages.
- Re-evaluation of LV function in patients with established cardiomyopathy when there has been a documented change in clinical status or to guide medical therapy.
In the Evaluation of HCM
- Initial evaluation of known or suspected HCM.
- Re-evaluation of known HCM in a patient with a change in clinical status to guide or evaluate therapy.
HCM, hypertrophic cardiomyopathy; LV, left ventricle; RV, right ventricle.
(Adapted from Douglas PS, Garcia MJ, Haines DE, et al. The ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166).- A murmur in a patient with cardiorespiratory symptoms.
M-Mode, Two-dimensional, and Three-dimensional Echocardiography
Best Imaging Planes
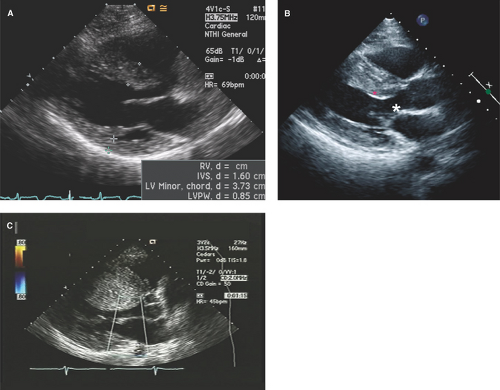
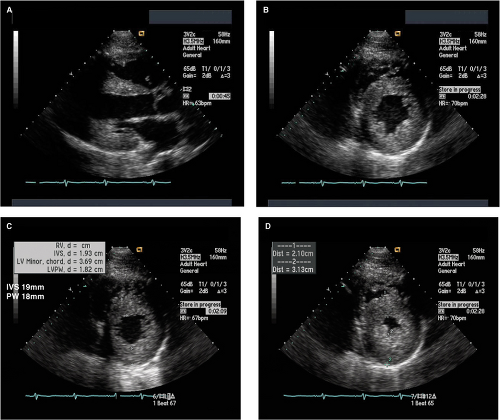
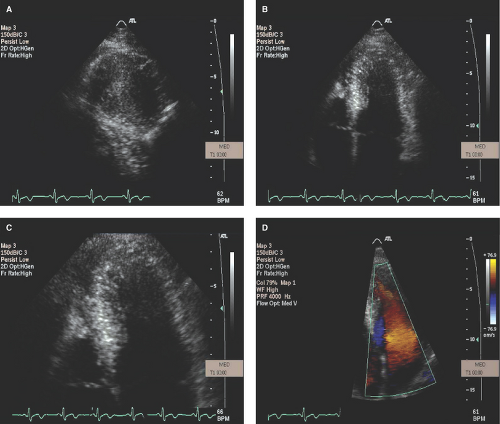
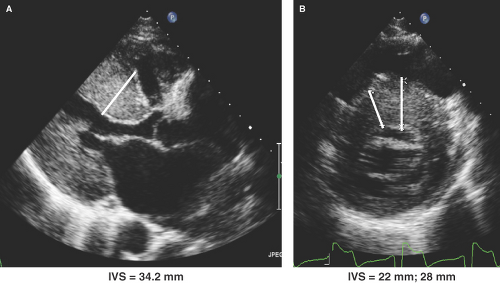
Parasternal long- and short-axis images are best for obtaining both M-mode and two-dimensional (2D) echo imaging of the LV and mitral valve for assessment of SAM, measurement of LV wall thickness, and identifying the maximal site of LVH as well as determining if ASH or concentric LVH is present (Figs. 15.1 to 15.3). Apical LVH is best seen on the two-, three-, four-, or five-chamber apical views (Figs. 15.4 and 15.5).
Parasternal long- and short-axis M-mode or 2D transthoracic echo (TTE) views are generally used for measurement of LV wall thickness in end diastole (Fig. 15.6). The parasternal short-axis view (Fig. 15.6B) allows more precise assessment and identification of the right ventricular (RV) septal endocardium as well as for identifying the sites of insertion of the papillary muscles on the right side of the septum and, thus, their exclusion when measuring septal thickness.
Apical three-, four-, and five-chamber 2D TTE views are also used to assess pattern and magnitude of LVH and SAM (Figs. 15.4 and 15.5).
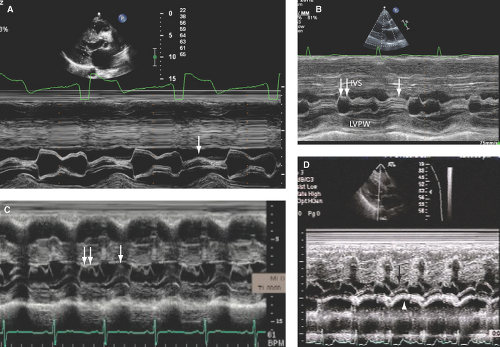
M-mode echo, due to its high temporal resolution, is useful to identify SAM and measure the duration of SAM-septal contact, which correlates with the magnitude of the LVOT gradient.
Parasternal 2D imaging can be useful to place M-mode cursor through the site of maximal SAM and/or SAM-septal contact (Fig. 15.7A–D). Patients vary in the degree and severity of SAM, and hypovolemia or an increase in the inotropic or contractile state will also increase the magnitude of SAM, duration of SAM-septal contact, and magnitude of LVOT gradient.
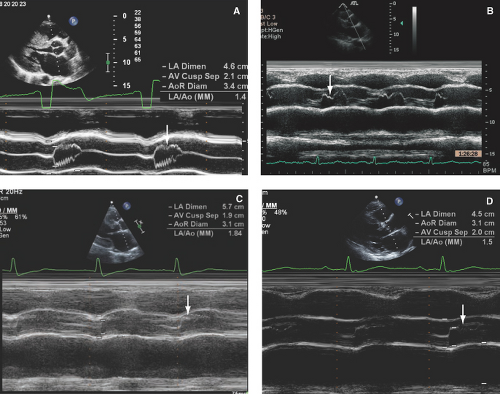
Figure 15.8: A-D: Four cases of varying severity of aortic systolic preclosure (arrows), with the most severe preclosure being seen in (B) from a patient with a 120 mmHg LVOT gradient and severe MR.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree