KEY POINTS
The acquired immunodeficiency syndrome (AIDS) is caused by chronic infection with the human immunodeficiency virus (HIV), which through its relentless replication causes progressive depletion of T-helper lymphocytes leading to severe cellular immunodeficiency.
In the absence of treatment, after a variable period, usually years from infection, multiple opportunistic infections or neoplasms characteristic of AIDS develop.
Combination antiretroviral therapy has been shown to prolong survival as well as disease-free interval. Furthermore, antiretroviral therapy has emerged as an effective primary prevention. Despite substantial progress in antiretroviral therapy, cure of the disease remains elusive.
Acute respiratory failure (ARF) secondary to Pneumocystis jirovecii pneumonia (PJP) is now less common than during the early years of HIV/AIDS as a cause of ICU admission among HIV-infected individuals with advanced HIV infection.
PJP usually is diagnosed in the ICU using bronchoalveolar lavage (BAL). BAL fluid should always be processed to allow identification of P jirovecii, fungi, common bacteria, mycobacteria, and viruses.
The mortality of PJP-related ARF has decreased substantially with the use of adjunctive systemic corticosteroids. Patients developing ARF despite corticosteroid treatment, however, continue to have a dismal prognosis.
Because of better treatment and prolonged survival, more patients are admitted to ICU who have HIV/AIDS as an underlying illness as opposed to the cause of ICU admission.
HIV cannot be transmitted through casual contact. Universal precautions, however, must be implemented and enforced routinely to minimize the risk of occupational exposure to HIV (as well as other infectious agents). The rate of seroconversion following a single accidental needle stick or mucous membrane exposure appears to be well below 1%.
The issue of life support should be discussed early and reassessed frequently with HIV-infected individuals. Because the outlook of AIDS and its related diseases has improved dramatically, rigid policies regarding ICU admission are not appropriate.
INTRODUCTION
It has been over three decades since the initial reports of unusual opportunistic infections and malignancies heralded the onset of the human immunodeficiency virus (HIV) epidemic.1,2 Over the last 30 years, our understanding of HIV transmission, pathogenesis, and viral replication has advanced considerably. The use of combination antiretroviral therapy (ART) has been shown to halt progressive immunologic decline with concomitant improvements in morbidity and mortality due to HIV-related acquired immunodeficiency syndrome (AIDS).3,4 As a result, survival rates among HIV-infected individuals who are able to access ART may begin to approximate those of the general population.5,6 In the United States, recent epidemiologic data suggest that mortality due to HIV infection has now dropped below that related to hepatitis C infection.7 Similar benefits have now also been demonstrated in resource-limited settings where ART programs have been implemented, such as in South Africa,8 Zambia,9 Uganda,10 and South East Asia.11,12
The proportion of AIDS cases requiring either hospitalization or admission to the ICU has declined since the introduction of ART in 1996. In recent years, HIV-related hospitalizations are less often due to opportunistic diseases compared with the pre-ART era. Management of patients presenting to the intensive care unit (ICU) should reflect these developments, and HIV infection alone should not affect decisions to pursue life-saving interventions among patients requiring ICU support. HIV-infected patients are now as likely to present with common reasons for admission such as trauma, drug-related overdose, or bacterial sepsis, as they are to present with HIV-related complications. That is, HIV/AIDS is now an important underlying condition as opposed to the cause of ICU admission. Physicians working in the ICU must remain informed regarding the management of common opportunistic infections that remain a cause of hospitalization for HIV-infected individuals. Physicians caring for critically ill patients who have HIV/AIDS must also become familiar with antiretroviral therapies and the need to avoid inadvertent harm due to treatment discontinuations, or toxicities arising due to drug-drug interactions.
HIV EPIDEMIOLOGY
There were an estimated 34 million individuals living with HIV/AIDS worldwide in 2010.13 The majority of individuals (22.9 million) reside in sub-Saharan Africa, with an estimated 2 to 3 million individuals living in North America and Europe. Globally, the incidence of HIV infection has declined from 3.0 million new infections in 2000 to 2.7 million in 2010. Within the United States, by the end of 2008 there were an estimated 1.1 million individuals living with HIV—of whom 20% are thought to be undiagnosed.14 Estimated 40 to 60,000 new diagnoses occur annually.14-16 The majority of diagnoses are occurring in men who have sex with men (MSM), with heterosexual transmission now accounting for approximately 30% of diagnoses.14 Since the widespread adoption of HIV screening of donated blood, parenteral transmission of HIV is limited almost exclusively to intravenous drug use. Finally, the infection can also be transmitted perinatally, although this is rare in a North American context. Uptake of ART has resulted in decreases in AIDS diagnoses and AIDS-related mortality (ie, patients are HIV positive without complications of AIDS) within the United States. Despite advances in therapy, a significant proportion of individuals estimated to be infected within the United States remain undiagnosed, linkage to care among HIV-infected individuals who are aware of their status remains incomplete, and accordingly such patients may present with common opportunistic infections and morbidities related to untreated HIV.
HIV PATHOGENESIS
Briefly, HIV preferentially infects T lymphocytes bearing the surface marker CD4, the so-called helper T cells. This tropism is mediated through a specific interaction between GP160, a viral envelope glycoprotein, and the CD4 molecule itself. HIV is also capable of infecting a number of other bone-marrow-derived cells, including monocytes, macrophages, Langerhans dendritic cells, and microglial cells.17,18
Once within the cell, the viral ribonucleic acid (RNA) and reverse transcriptase are released. The reverse transcriptase generates a deoxyribonucleic acid (DNA) sequence complementary to the viral RNA, which then integrates into the host cell’s genome to produce new viral particles, which in turn will infect other susceptible cells. Relentless HIV replication ultimately causes CD4 cell dysfunction and cell death, leading to severe cellular immunodeficiency.
The CD4+ lymphocyte count is thus regarded as one of the key surrogate markers for prognostic staging and therapeutic monitoring of HIV-infected individuals (see Table 69-1). A range of 400 to 1400 cells/mm3 (0.40-1.40 × 109/L) is considered normal in most laboratories. The CD4 count usually is reported as a fraction and an absolute count. Although the absolute CD4 count is usually a good reflection of the degree of immunodeficiency in a given patient, it must be noted that under specific circumstances this may be misleading. It is therefore advisable to monitor the CD4 percentage to ensure that this is in general agreement with the absolute CD4 count. Absolute CD4 counts are used widely to guide therapeutic decisions regarding the use of antiretrovirals and preventive strategies, yet they are subject to considerable variability. CD4 counts show circadian variation, which is lowest in the morning and highest in the evening. In normal individuals, the evening CD4 cell count can be nearly double the morning nadir. Despite controlling the time of collection, HIV-infected individuals who are stable clinically will still show considerable variation in CD4 counts. Short-term CD4 count fluctuations of nearly 30% may occur that are not attributable to a change in disease status. In addition, acute infection or illness may lead to a transient decline in absolute CD4 cell count with preserved CD4 percentage, a finding also associated with advanced liver disease.19,20
Common Laboratory Evaluation of the HIV-Infected Patient
| Test | Comment |
|---|---|
| HIV-specific tests | |
| Plasma HIV RNA (viral load) | |
| CD4+ lymphocyte count (absolute and percentage) | |
| Baseline HIV resistance testing (HIV genotype) | |
| HLA-B5701 assay | Presence of this marker is associated with increased risk of abacavir hypersensitivity reaction |
| Coinfection/opportunistic diseases assessment | |
| Serologic testing for syphilis | Rapid plasma reagin (RPR) |
| Serologic testing for hepatitis A, B, and C | |
| Toxoplasma serology | Disease seen in those with CD4 <100 |
| Serum cryptococcal antigen | Sensitive screen for cryptococcal meningitis |
| Mycobacterial blood cultures | Disseminated mycobacterium avium complex seen with CD4 <50 |
| CMV | Retinitis screen with CD4 <50 |
Overall, it is important to monitor the trends in CD4 counts over time to avoid placing too much emphasis on the specific number derived from a single determination. Despite these limitations, the CD4 count remains a valuable tool when attempting to establish the differential diagnoses in a given patient. For example, it would be very unusual (although still possible, particularly in the context of immune reconstitution inflammatory syndromes) to have a case of Pneumocystis jirovecii, Mycobacterium avium complex (MAC), or cytomegalovirus (CMV) disease with a CD4 count within the normal range.
Plasma viral load has been shown to be an independent predictor of disease progression and death in untreated HIV-infected individuals.21
NATURAL HISTORY
Acute HIV infection is associated with retrospectively identified transient symptomatic illness in 40% to 90% of patients.22 This is most often a nonspecific flu-like illness often confused with acute infectious mononucleosis and characterized by fever (>80%-90%), fatigue (>70%-90%), rash (>40%-80%), headache (32%-70%), lymphadenopathy (40%-70%), pharyngitis (50%-70%), and aseptic meningitis (24%), as well as other symptoms. This usually known as seroconversion illness, is believed to be an immune-complex-mediated phenomenon resulting from early antibody response to the infection by HIV. Typically, after a variable period (rarely less than 2 years) with few or no symptoms, progressive immunodeficiency develops, rendering the individual susceptible to the development of opportunistic infections, wasting, and/or neoplasms characteristic of AIDS. AIDS remains incurable despite considerable progress in antiretroviral therapy. The often prolonged period of clinical latency is characterized by continued viral replication and decline of the immune system, as illustrated by the progressive destruction of the lymph node architecture.23
ANTIRETROVIRAL THERAPY IN THE ICU
Combination therapy regimens have been shown to prolong survival and the disease-free interval substantially among HIV-infected individuals. Therapeutic guidelines issued by national and international organizations for the management of HIV-infected individuals have evolved substantially in response to findings from clinical trials, cohort studies and pathogenesis research, and are updated on a regular basis.24-27
Individuals with symptomatic disease or AIDS-defining conditions require initiation of ART. With the recognition that the inflammatory consequences of unchecked HIV replication may serve as a driver of non-AIDS-defining clinical events, current guidelines support early initiation of therapy in asymptomatic individuals.28,29 At present, there is broad consensus that therapy should be initiated at an absolute CD4+ cell count threshold of 350 cells/mm3 and is recommended at thresholds <500 cells/mm3 in most guidelines.24,25 In addition, the presence of other coinfections such as active hepatitis B, HIV-associated nephropathy, HIV-associated neurocognitive impairment, and preexisting coronary artery disease are recognized as conditions that would benefit from initiation of ART regardless of CD4 cell count.25,27,30
There are six major classes of antiretroviral therapy, targeting virtually all aspects of the viral lifecycle of the HIV including CCR5 coreceptor, reverse transcriptase inhibitors, integrase inhibitors, and protease inhibitors (PI). Commonly available agents within each drug class are listed in Table 69-2.
Common Antiretroviral Agents by Drug Class25
| Class | Agent | Common Side effects/Comments |
|---|---|---|
| Nucleoside reverse transcriptase inhibitors (NRTIs) | Zidovudine (AZT) | Bone marrow suppression: macrocytic anemia, leucopenia. GI intolerance, headache, insomnia, lactic acidosis, and hepatic steatosis and myositis (with elevated CPK). An IV formulation is available |
| Didanosine (DDI) | Pancreatitis, lactic acidosis, peripheral neuropathy, hypertriglyceridemia, hyperuricemia, gout, lactic acidosis, and hepatic steatosis | |
| Stavudine (D4T) | Pancreatitis, lactic acidosis, sensory peripheral neuropathy, neuromuscular weakness, and hepatic steatosis | |
| Lamivudine (3TC)a | A liquid formulation is available | |
| Emtricitabine (FTC)a | Skin color change, rash | |
| Tenofovira | Renal impairment—classically Fanconi syndrome with proximal tubular injury | |
| Abacavira | Hypersensitivity reaction—requires prescreening with HLA B5701 assay | |
| Hypersensitivity reaction may be fatal (symptoms may include fever, rash, fatigue, vomiting, abdominal pain, diarrhea, cough, shortness of breath). If hypersensitivity diagnosed, then abacavir should be stopped and never restarted | ||
| A liquid formulation is available | ||
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | Efavirenza | Potentially teratogenic |
| Neuropsychiatric side effects common in first 4 weeks. Rash, central nervous system symptoms (eg, abnormal dreams, confusion, agitation, hallucinations), hepatitis. Drug interactions caused by induction of cytochrome P450 | ||
| Nevirapine | Hypersensitivity reaction. Rash, hepatitis. Drug interactions caused by induction of cytochrome P450. Should not be used in men with CD4 >400 and women with CD4 >250 cells/mm3 | |
| Etravirine | Rash, GI intolerance, rare severe hypersensitivity (DRESS syndrome). | |
| Rilpivirine | Central nervous system symptoms occur infrequently compared to efavirenz, headache, rash, lipid abnormalities, transaminase elevations. Should not be used if viral load >100,000 copies/mL. | |
| Protease inhibitors (PIs) | Atazanavira | Requires acid environment for absorption—concomitant proton-pump inhibitor therapy should be avoided |
| These require boosting by ritonavir and as such potential drug interactions caused by inhibition of cytochrome P450 must be considered | Asymptomatic hyperbilirubinemia, QT-interval prolongation, possible increased bleeding episodes in hemophilia | |
| Darunavira | GI intolerance, headache, fatigue, hypertriglyceridemia. Potential cross-reactivity in severe sulfa allergy | |
| Lopinavir | Only agent coformulated with ritonavir. Diarrhea, headache, hyperlipidemia, diabetes | |
| A liquid formulation is available | ||
| Saquinavir | GI intolerance, headache, transaminase elevation, possible increased bleeding episodes in hemophilia | |
| Fosamprenavir | GI intolerance, rash, hyperlipidemia | |
| Integrase inhibitors | Raltegravira | Few side effects, but BID dosing recommended |
| Elvitegravir | Requires boosting with cobicistat | |
| Entry inhibitors | Enfuvirtide | Administered subcutaneously |
| Injection site reactions, pneumonia, eosinophilia | ||
| CCR5 antagonists | Maraviroc | Requires prescreening with viral tropism assay to evaluate if CCR5 coreceptor is utilized in binding process. Diarrhea, anemia, rash, depression, transaminase elevation |
Recommended regimens for first-line therapy are now either once or twice daily, and most patients have few side effects or toxicities. The currently recommended regimens include the combination of two nucleoside reverse transcriptase inhibitor (NRTI) agents (usually tenofovir/emtricitabine or abacavir/lamivudine as coformulated tablets) with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) such as efavirenz or rilpivirine or a protease inhibitor (atazanavir or darunavir) or an integrase inhibitor (raltegravir or elvitegravir). Protease inhibitors (PIs) commonly require pharmacokinetic boosting with ritonavir, a potent cytochrome P450 3A4 inhibitor. The use of low-dose ritonavir allows for extended dosing intervals for the primary protease inhibitor (once or twice daily dosing) with associated improvements in pill burden and adherence.31 Low dose ritonavir also increases potency and decreases likelihood of PI resistance compared to unboosted PIs.32,33 Elvitegravir is combined with a novel boosting agent namely cobicistat which again acts as a cytochrome P450 inhibitor.34,35
When to Start/Discontinue Antiretroviral Therapy: Initiation of antiretroviral therapy is rarely required on an urgent basis within the ICU for individuals newly diagnosed or not previously treated. In most circumstances, baseline HIV-related laboratory work (Table 69-1) can be done to then determine an optimal regimen. Consultation with an HIV-experienced physician/service is very helpful in selecting appropriate therapy and to provide follow-up after ICU discharge for long-term management.
In circumstances in which the individual presents with an opportunistic infection, early initiation of ART is desirable. In the AIDS Clinical Trials Group (ACTG) 5164 trial, 282 HIV-infected individuals with acute opportunistic infections were randomized to receive early (within 14 days of starting appropriate management for the infection) compared to delayed ART (initiated only after completion of therapy for the infection).36 Overall 63% of individuals had Pneumocystis pneumonia as the underlying infection. Patients randomized to early ART (ART was started a median 12 days after initiation of antimicrobial therapy directed at the opportunistic infection) had significantly fewer AIDS progression events and deaths (odds ratio [OR] = 0.51; 95% CI = 0.27-0.94) and a greater time to AIDS progression or death (stratified hazard ratio [HR] = 0.53; 95% CI = 0.30-0.92).36 There was no difference between early and delayed ART in adverse events; that is, early ART did not significantly increase rates of adverse events. In cases in which tuberculosis is the opportunistic infection, results of early observational studies support early initiation of ART in individuals with CD4 cell counts <100 cells/mm3.37 This observation and recommendation has subsequently been validated in several randomized controlled trials, which clearly demonstrate the benefits of early initiation of ART within 2 weeks of tuberculosis therapy, particularly in patients with CD4 cell counts <50 cells/mm3.38-40 In patients with tuberculosis and higher CD4 cell counts, antiretroviral therapy should be initiated within 8 to 12 weeks of starting antituberculous therapy. One important potential risk of early therapy is the development of immune reconstitution inflammatory syndrome (discussed below).
Commonly, patients with underlying HIV are on antiretroviral therapy when admitted to the ICU. It is important to remain open to the possibility of new and previously unrecognized adverse effects of these therapies. Use of antiretroviral therapy in the ICU poses concerns and questions regarding potential drug-drug interactions, poor absorption of medications in critically ill patients, and the risk of ART resistance if antiretroviral medications are abruptly discontinued. Agents such as the NNRTI class are known to have longer pharmacologic half-life, leading to essential monotherapy if the ART regimen is stopped abruptly. This period of monotherapy is associated with the development of drug resistance41 and therefore, replacement with a protease inhibitor is recommended.42 If a regimen is to be discontinued due to toxicity, then the offending agent should be substituted with another agent if possible. If the entire NNRTI regimen is to be discontinued, a staggered stop in which the nucleoside backbone is continued for an additional 7 to 10 days can be considered.24
Drug-Drug Interactions: Both the NNRTI and PI classes are active at the level of the cytochrome P450 isoenzyme system, with the potential to act as either potent inducers or inhibitors of metabolism of many other medications. Conversely levels of NNRTI and PI may be altered by the cytochrome P450 isoenzyme system action of other medications used in the ICU setting. Accordingly, specific antiarrythmics, antihistamines, and some benzodiazepines may be contraindicated. Antiseizure medications pose particular concern, as do rifampin, statins (simvastatin and lovastatin are contraindicated), and even inhaled corticosteroids. Inhaled corticosteroids may induce steroid excess or adrenal suppression.43,44 Use of acid-lowering therapies may alter absorption of the PI atazanavir and the NNRTI agent rilpivirine.
Immune Reconstitution Inflammatory Syndrome: Suppression of HIV replication through the use of ART, and the associated CD4 cell count increase, occasionally causes exaggerated inflammatory responses to newly recognized antigens. This phenomenon has been named immune reconstitution inflammatory syndrome (IRIS). The current understanding of the pathophysiology of IRIS includes interactions between immune recovery, previously unrecognized (subclinical or residual) antigenic burden, and possible host genetic variation.45 Among HIV-negative patients, this phenomenon occurs occasionally during the course of chronic hepatitis B and during treatment for borderline lepromatous leprosy (reversal reaction, Lepra type I) or tuberculosis (eg, central nervous system tuberculomas) and is due to improvement in cell-mediated immunity.46 IRIS has been described as both an unmasking of subclinical latent infection or a worsening of already documented preexisting disease. IRIS has been well described in the context of CMV retinitis, tuberculosis (MTb), MAC, Pneumocystis jirovecii pneumonia (PJP), cryptococcosis, progressive multifocal leukoencephalopathy, and herpes zoster infection.45,47
Proposed criteria for diagnosis include documented viral load decreases and new or worsening symptoms of an infectious or inflammatory condition after initiation of ART.48 The differential diagnosis may include adverse drug effects and coexisting unrecognized infections (nosocomial or community acquired). Some opportunistic infections show atypical features in the context of IRIS, particularly MAC, CMV retinitis, and cryptococcal meningitis.49,50 For preexisting opportunistic infections, the diagnosis of IRIS should be consistent with published case definitions, but to some extent is one of exclusion, including consideration of coexisting opportunistic infections and also drug-resistant opportunistic infections. MAC IRIS is usually an unmasking of subclinical infection in the setting of a virologic response to ART, and when the organism is recovered from a normally sterile body site, the diagnosis is established. IRIS is important to consider in the differential diagnosis of any HIV-infected patient who appears to worsen during the course of therapy for tuberculosis or PJP after initiation of antiretroviral therapy. M tuberculosis immune reconstitution syndrome (MTb-IRIS) is a paradoxical worsening of the signs and symptoms of tuberculosis during the course of antituberculous therapy. MTb-IRIS has been reported to occur in up to 36% of HIV-infected patients who initiate ART.51 The manifestations of MTb-IRIS may include fever, worsening pulmonary infiltrates, lymphadenopathy, and CNS granulomas. Case definitions for MTb-IRIS have been developed and help appropriately classify cases.52
Initiation of combination antiretrovirals during therapy for PJP has been associated with a paradoxical worsening of the pulmonary infiltrates and lung function in up to 5% to 18% of patients.53 Among 17 patients with PJP immune reconstitution syndrome, the clinical worsening was observed 3 to 17 days after starting the antiretroviral regimen. Flow cytometry of BAL specimens in such patients may show a higher CD4/CD8 ratio than usually observed in PJP owing to an influx of CD4 cells during immune reconstitution.54 Transbronchial lung biopsy may reveal a prominent alveolar infiltrate consisting of lymphocytes, macrophages, and neutrophils with few or no demonstrable PJP organisms.55 The diagnosis is established by bronchoscopy and transbronchial biopsy in order to demonstrate the above-mentioned findings and exclude other possible opportunistic diseases.
IRIS has been described in patients with cryptococcosis who subsequently receive ART.56 Presentations may include pulmonary or soft tissue lesions that often are culture negative but demonstrate organisms consistent with cryptococcus on smear or histology. Others may experience an exacerbation of cryptococcal meningitis associated with a negative CSF culture and a higher than usual CSF pleocytosis.50 The optimal timing for initiation of ART in the setting of cryptococcal meningitis is unclear because of concerns of risk of potentially life-threatening IRIS. A randomized trial in Africa showed that concurrent initiation of both antifungal therapy and ART (within 72 hours) increased mortality.57 Current guidelines suggest a minimum 2-week course of antifungal therapy; however, a longer duration (4-6 weeks) may be necessary. Clinical trials are ongoing to address this issue.
Clinical management of IRIS usually includes nonsteroidal anti-inflammatory agents or corticosteroids. A randomized clinical trial supports the role of corticosteroids in the management of MTb-IRIS.58 Timing of initiation of antiretroviral therapy in the context of IRIS is discussed above. If antiretroviral therapy has already been initiated, it should be continued unless life-threatening features are present.45
CHANGING SPECTRUM OF ICU PRESENTATIONS AMONG HIV-INFECTED PATIENTS IN THE ART ERA
In retrospective series, approximately 5% to 12% of hospitalized HIV-infected patients require ICU support.59 Admissions in the pre-ART era were driven predominantly by opportunistic infections, notably Pneumocystis jirovecii pneumonia. Causes of admissions in the ART era are generally unrelated to HIV infection and are now very similar to the general non-HIV ICU population of comparable age and risk groups. Patients with these conditions usually respond to standard management, and their prognosis appears to be similar to that of non-HIV-infected patients who have the same condition unless there is concomitant severe immunodeficiency, in which case the prognosis tends to be determined by the severity of the immunodeficiency. Overall, survival of HIV-infected patients in the ICU has also improved, with over 70% surviving to hospital discharge.60
Acute respiratory failure (ARF) remains the most common reason for admission. Community-acquired bacterial pneumonia and complications of chronic obstructive lung disease are common reasons for ICU admission of HIV-infected patients, while PJP accounts for 3% to 12% of cases.60 Sepsis due to bacteria and other bacterial infections such as endocarditis remain important etiologies of ICU admission and morbidity among HIV-infected individuals, particularly those with comorbid injection drug use.61 The mean age of HIV-infected individuals has increased because of better chronic HIV management. Comorbidities related to the aging of the HIV-infected population such as cardiovascular disease or end-stage liver disease (due to hepatitis C coinfection) are also common reasons for need for ICU support. Other conditions, such as cerebral toxoplasmosis, gastrointestinal bleeding, Kaposi sarcoma, lymphoma, and other malignancies account for the remaining HIV-related ICU admissions. The broad differential diagnosis of opportunistic infections and diseases should be kept in mind to avoid delays in diagnosis or misdiagnosis.
Less frequently, patients with AIDS may be admitted to the ICU without prior knowledge of their HIV status. Certainly, the presence or history of minor or major opportunistic infections, wasting, otherwise unexplained extensive herpes zoster, or persistent generalized lymphadenopathy combined with a history (or clinical evidence) of high-risk activities must trigger consideration of HIV infection in the differential diagnosis. Furthermore, laboratory abnormalities found commonly among HIV-infected individuals can provide the clues to consider HIV infection. Common laboratory abnormalities in HIV-infected patients include lymphopenia, anemia, thrombocytopenia, and hypergammaglobulinemia. It must be emphasized, however, that HIV infection should not be diagnosed unless HIV has been confirmed using specific serologic tests, most commonly, enzyme-linked immunosorbent assay (ELISA) and Western blot. We reemphasize that universal precautions must be followed by all clinical staff caring for ICU (and indeed all hospitalized) patients.
APPROACH TO THE HIV-INFECTED PATIENT IN THE ICU
Pulmonary and radiologic manifestations of HIV infection are diverse and include both infectious and noninfectious conditions (see Table 69-3).62 Bacterial pneumonias remain most common causes of pulmonary infection and cause considerable morbidity and mortality worldwide.63,64 The most common etiology of bacterial pneumonia in HIV-infected individuals is Streptococcus pneumoniae, followed by Haemophilus influenzae.63,65,66 Other bacterial agents identified in the setting of HIV-related bacterial pneumonia include Pseudomonas aeruginosa, Staphylococcus aureus, and less commonly Legionella pneumophila.65,67-69
Major Radiologic Differential Diagnosis of Lung Involvement in AIDS
| Normal chest x-ray | PJP, MAC, MTb |
| Diffuse or localized interstitial pattern | PJP, MTb, CMV, ALI/ARDS, cryptococcosis |
| Diffuse alveolar pattern | PJP, viral pneumonia, cryptococcosis, cardiogenic pulmonary edema, ALI/ARDS, VALI, ventilator-associated pneumonia |
| Miliary pattern or reticulonodular | MTb, histoplasmosis, coccidiodomycosis, cryptococcosis |
| Consolidation | Common bacteria, MTB, PJP, KS, ventilator-associated pneumonia |
| Nodular opacity | MTb, cryptococcosis, histoplasmosis, coccidioidomycosis, common bacteria, KS, lymphoma, carcinoma |
| Upper lung field involvement | PJP, MTb |
| Pneumothorax | PJP, ventilator-associated barotrauma |
| Cavity | Bacteria, MTb, aspergillosis, histoplasmosis, coccidioidomycosis, PJP |
| Pleural effusion | Common bacteria, mycobacteria, KS |
The rates of hospitalization for pneumonia have decreased. Population-based national data from Denmark demonstrated that hospitalization rates for pneumonia among HIV-infected individuals have decreased in the ART era, dropping 50.6 hospitalizations per 1000 person-years during 1995-1996 to 19.7 hospitalizations per 1000 person-years during 2005-2007.70 Nosocomial bacterial pneumonias among HIV-infected individuals are indistinguishable from those occurring in other hospitalized patients. These are usually caused by gram-negative organisms and tend to have a high mortality despite appropriate therapy.
Ventilator-associated pneumonia (VAP) may complicate the course of HIV-infected patients who require mechanical ventilation. There is an increasing frequency of S aureus (methicillin-resistant S aureus [MRSA] or methicillin-sensitive S aureus [MSSA]) as a cause of VAP. In addition, aerobic gram-negative bacilli remain common causes of VAP. The other diagnosis to consider in patients who have pulmonary infiltrates and who require mechanical ventilation is ventilator-associated lung injury. The details of mechanical ventilation are discussed in Chaps. 48, 49, 51, and 52. HIV-infected patients who have acute respiratory distress syndrome should receive lung-protective ventilation.71
Clinical features of community-acquired or ventilator-associated bacterial pneumonia are indistinguishable from those described in the immunocompetent host. Chest radiographs usually demonstrate segmental or lobar consolidation.
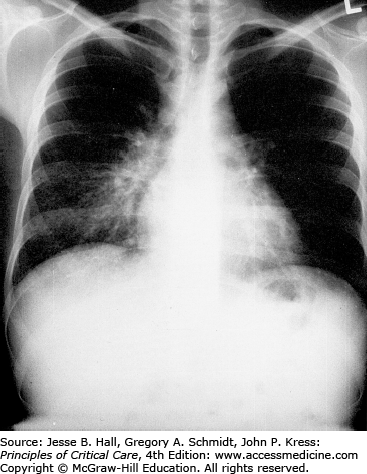
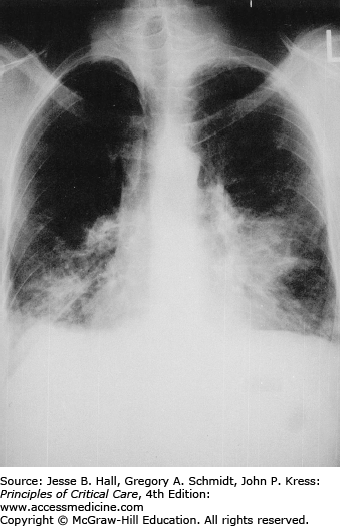
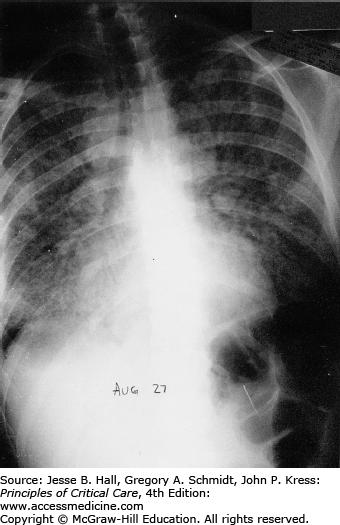
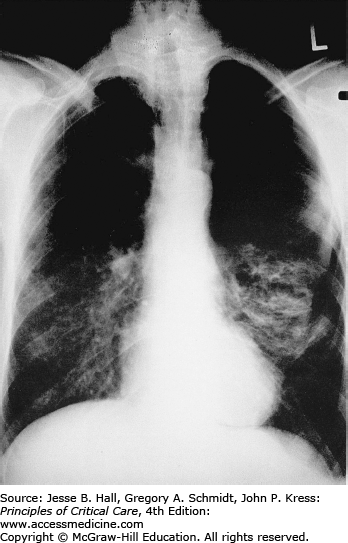
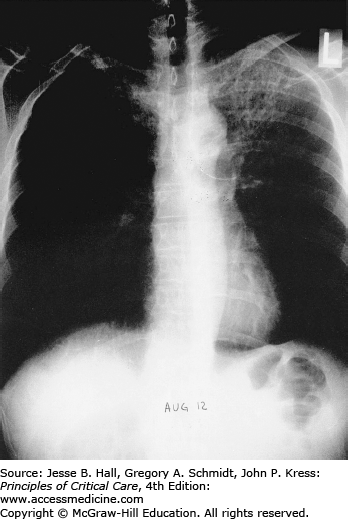
Opportunistic infections and malignancies should be considered when the CD4 cell count is below 200 cells/mm3 although occasionally PJP may present at higher CD4 thresholds. Initial therapy for pneumonia should include broad-spectrum antimicrobial therapy and empiric influenza coverage should also be considered during winter. Empiric coverage for PJP is not unreasonable if the radiographic findings are supportive (see Fig. 69-1).
Initial workup should include sputum culture and sensitivity for bacteria, fungi, and mycobacteria. Blood cultures should also be obtained, and in selected patients (ie, in those with advanced disease and CD4 cell counts below 50 or 100 cells/mm3), mycobacterial and fungal blood cultures should be obtained. The key to diagnosis often requires suction for tracheobronchial secretions. In the ICU setting, particularly in ventilated patients, bronchoscopic bronchoalveolar lavage (BAL) is the preferred approach. Experienced specialists should perform BAL because critically ill patients with pneumonia are often at risk of complications from bronchoscopy and BAL (such as hypoxemia and hypotension) and because of the need for care regarding the handling of specimens. In the few patients for whom the initial BAL does not provide a diagnosis, a transbronchial or open lung biopsy should be considered. However, the appropriateness of such intervention is best decided on a case-by-case basis after careful assessment of the general status of the patient, as well as the likelihood of diagnosing a treatable condition.
PNEUMOCYSTIS JIROVECII PNEUMONIA
Pneumocystis jirovecii (formerly carinii), recently reclassified as a fungus but having some properties of protozoa, is a ubiquitous organism that produces human disease throughout the world, usually in the setting of severe immunosuppression.72,73 Asymptomatic primary infection generally occurs early in life. Rarely, P jirovecii can be found incidentally at autopsy in the absence of symptoms. It is not clear whether this represents late infection or early disease not yet manifested clinically.
Pneumocystis remains an important pulmonary pathogen, predominantly among individuals who were unaware of their HIV status prior to presentation, or among individuals nonadherent to either prophylactic strategies or ART in general.74 Rates of PJP were high in the pre-ART era, with an incidence of 20 cases per 100 person-years in patients with CD4 cell counts less than 200 cells/mm3.75 The incidence of PJP declined markedly after the introduction of ART. Rates of PJP in the United States declined 21.5% per year from 1996 to 1998.76 Similarly, in the EuroSIDA cohort, the incidence of PJP declined from 4.9 cases per 100 person-years prior to the introduction of ART to 0.3 cases per 100 person-years in 1998.77
PJP presents initially as a subacute condition, with a history of progressive exertional dyspnea accompanied by fever and cough. Occasionally a more acute illness with progression over the span of a several days may be seen. Acute dyspnea with chest pain may be indicative of a pneumothorax. In critically ill patients, the physical examination usually demonstrates evidence of acute respiratory distress, with surprisingly few adventitious sounds on auscultation of the chest. Acute hypoxemic respiratory failure requiring mechanical ventilation has been reported to occur in as many as 20% of hospitalized patients.78 Most often this occurs within the first 3 days of starting antimicrobial therapy; less frequently acute hypoxemic respiratory failure develops as a complication of diagnostic bronchoscopy and rarely as the initial presentation to the emergency room.78
Classically, radiographic imaging demonstrates bilateral interstitial infiltrates; however, other presentations are possible including cystic changes, pneumothoraces, nodular or masslike opacities, and even cavities.79 Clinically overt PJP usually develops over a period of several days to weeks, and in this time, the radiologic picture tends to progress from a normal chest radiograph to a diffuse bilateral interstitial pattern. Varying degrees of alveolar involvement may also be seen; even frank consolidation may occur, as seen in Figures 69-1 through 69-4. Upper lung field involvement, as seen in Figure 69-5, has also been recognized increasingly, particularly (but not exclusively) in the context of aerosol pentamidine prophylaxis. To what extent aerosol pentamidine prophylaxis is responsible for the apparent increased frequency of PCP-related pneumothoraces remains controversial. Hypoxemia is indicative of more severe disease.
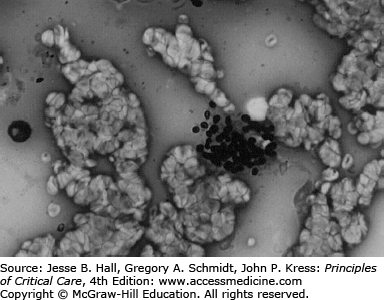
Laboratory abnormalities may include elevated lactate dehydrogenase, although this is not diagnostic. Bronchoscopy with bronchial brushings and BAL can establish the diagnosis. BAL is a rapid, safe, and effective means of obtaining tracheobronchial secretions to provide an adequate diagnostic specimen. Unlike in non-HIV cases of PJP, lung biopsy is seldom required to confirm the diagnosis of PJP in those with HIV, because of a greater organism load. Organisms can be demonstrated by staining with either toluidine blue, methenamine silver, or Giemsa stain (see Fig. 69-6).
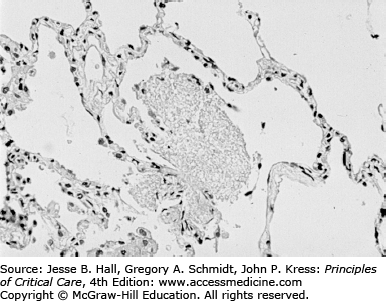
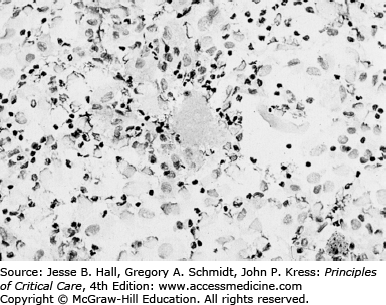
As seen in Figure 69-6, the usual pathologic picture of PJP consists of a mild to moderate interstitial inflammatory reaction with predominance of lymphocytes and alveolar macrophages and the presence of a foamy alveolar exudate (as seen with hematoxylin and eosin [H&E] staining). The foamy appearance of the alveolar exudate is caused by the presence of the cystic form of the organism, which is not stained with H&E but can be easily recognized using readily available special stains (Figs. 69-7 and 69-8). As seen in Figure 69-8, BAL allows clear identification of the organism if the specimen is concentrated and stained appropriately.
The composition of the alveolar exudate has not been established conclusively. However, BAL studies suggest that this is an inflammatory exudate rich in immunoglobulins, macrophages, and suppressor cytotoxic lymphocytes.80 Although P jirovecii infection is usually confined to the lungs, systemic pneumocystosis (involving liver, spleen, lymph nodes, adrenals, and eyes) has been reported occasionally.81 Polymerase chain reaction (PCR) methodology has been applied to the diagnosis of PJP using blood, sputum, and BAL but is not widely available and remains investigational.
Trimethoprim-sulfamethoxazole (TMP-SMX) is effective against P jirovecii, as well as various gram-negative and gram-positive bacterial organisms. Intravenous trimethoprim-sulfamethoxazole (15 mg/kg of the trimethoprim component divided three times daily) is recommended for severely ill patients (eg, <SPAN role=presentation tabIndex=0 id=MathJax-Element-1-Frame class=MathJax style="POSITION: relative" data-mathml='PaO2′>PaO2PaO2 <70 mm Hg, A-a gradient >45 mm Hg).82 The optimal duration of therapy is 21 days (see Table 69-4). Side effects of trimethoprim-sulfamethoxazole include rash (including severe mucocutaneous reactions), cytopenias, and renal dysfunction. A number of reports have documented successful desensitization of TMP-SMX-allergic patients using progressively larger doses of the drug. Hypersensitivity-type reactions such as fever or rash can also be treated with diphenhydramine or corticosteroids.83
Antimicrobial Therapy of Common Infections in AIDS Patients
| Infection | Drug of Choice | Total Daily Dose | Dose Interval | Route | Usual Duration | Alternative Therapy |
|---|---|---|---|---|---|---|
| Protozoa | ||||||
| Toxoplasmosis (Toxoplasma gondii) | Pyrimethaminea | 200 mg loading dose, then 50 mg (if <60kg), or 75 mg (if >60kg) | Daily | PO | ≥6 weeksb | Pyrimethamine (leucovorin) plus clindamycin 600 mg IVc or PO q6h, |
| Or | ||||||
| TMP-SMX (TMP 5 mg/kg and SMX 25 mg/kg) IV or PO BID | ||||||
| plus | ||||||
| Sulfadiazine | 4000 mg (if <60kg), or 6000 mg (if >60kg) | 6h | PO | >6 weeks | ||
| plus | 10-25 mg | |||||
| Leucovorin | Daily | PO | >6 weeks | |||
| Maintenance therapy: | ||||||
| Pyrimethaminea | 25-50 mg | Daily | PO | Indefinitely | Pyrimethaminea 25-50 mg/d PO | |
| plus | plus leucovorin 10-25 mg PO daily | |||||
| plus clindamycin 600 mg PO q8h | ||||||
| Or | ||||||
| TMP-SMX DS 1 tablet BID | ||||||
| Sulfadiazine | 2000-4000 mg | 12h | PO | Indefinitely | ||
| plus | ||||||
| Leucovorin | 10-25 mg | Daily | PO | Indefinitely | ||
| Cryptosporidiosis (Cryptosporidium) | No proven effective therapy | Nitazoxanide 500-1000 mg PO BID for 14d, or Paromomycin 500 mg PO QID for 14-21 d (optimize antiretroviral therapy, rehydrate) | ||||
| Rehydration and electrolyte replacement | ||||||
| Optimize antiretroviral therapy | ||||||
| Isosporiasis (Isospora belli) | Trimethoprim-sulfamethoxazole | 640 mg | ||||
| 3200 mg | 6h | PO, IV | 10dd | Pyrimethamine 50-75 mg PO daily plus leucovorin 5-10 mg PO daily for 4 weeks; or ciprofloxacin 500 mg PO BID × 7d | ||
| Maintenance therapy: | ||||||
| Trimethoprim, 160 mg, sulfamethoxazole, 800 mg | 3 times per week | PO | Until CD4 >200 for >6 months | Pyrimethamine 25 mg PO daily plus folinic acid 5 mg PO daily | ||
| Pneumocystosis (P jirovecii) | Intravenous therapy | |||||
| TMP-SMX | TMP 15-20 mg/kg and SMX 75-100 mg/kg | 6-8h | IV | 21d | Clindamycin 600 mg q6h IV plus primaquine 30 mg (base) daily PO | |
| or | ||||||
| Pentamidine 4 mg/kg per day IV | ||||||
| Oral therapy | ||||||
| TMP-SMX | TMP 15-20 mg/kg and SMX 75-100 mg/kg | 8h | PO | 21d | TMP-SMX 2 DS tablets q8h PO; | |
| or dapsone 100 mg daily plus TMP 5 mg/kg PO TID | ||||||
| or clindamycin 450 mg q6h PO plus primaquine 30 mg (base) daily | ||||||
| or atovaquone 750 mg q12h PO with food | ||||||
| Primary Prophylaxis or Maintenance therapy: | ||||||
| TMP-SMX | 1 DS tablet (preferred) | Daily | PO | Indefinitely | Dapsone 100 mg PO daily, | |
| Or 1 SS tablet | Or atovaquone 1500 mg PO daily | |||||
| Candidiasis oropharyngeal | Fluconazole | 100 mg | Daily | PO | 7-14d | Itraconazole 100 mg PO daily for 14de (or 100 mg BID for 7d); or topical antifungal (nystatin or clotrimazole 3-5 times daily) |
| Esophageal | Fluconazole | 100-400 mg | Daily | PO/IV | 14-21d | Itraconazole 200 mg PO daily (3 weeks)e |
| or Voriconazole 200 mg PO/IV BID, | ||||||
| or Posaconazole 400 mg PO BID, | ||||||
| or an echinocandin, | ||||||
| or amphotericin B formulation | ||||||
| Fluconazole refractory mucosal candidiasis (oral esophageal) |