An estimation of cardiovascular function based on the physical examination and standard hemodynamic data such as blood pressure, central venous pressure, and urine output may be discordant from measured values of cardiac function, cardiac output, and tissue oxygenation.
An estimation of cardiovascular function based on the physical examination and standard hemodynamic data such as blood pressure, central venous pressure, and urine output may be discordant from measured values of cardiac function, cardiac output, and tissue oxygenation. The complementary use of hemodynamic monitoring technology enables the clinician to make a more timely and accurate assessment of cardiovascular function.
The complementary use of hemodynamic monitoring technology enables the clinician to make a more timely and accurate assessment of cardiovascular function. One of the tenets of critical care medicine is to ensure adequate tissue oxygenation. This determination is often based on the physical examination and the interpretation of standard hemodynamic parameters such as blood pressure, central venous pressure (CVP), and urine output. However, studies have demonstrated significant discordance between assessments based on these parameters and those based on measurements of cardiac function, cardiac output (CO), and tissue oxygenation (1,2,3,4,5,6,7,8). The pediatric intensivist must appreciate that no single observation or measurement taken in isolation can provide adequate hemodynamic monitoring. Rather, it is the application of a range of observations and measurements that allows the care team to balance oxygen delivery with the child’s metabolic demands. The type and intensity of hemodynamic monitoring should be calibrated to prevent tissue hypoxia episodes and, when necessary, to document that adequate tissue oxygenation has been restored.
One of the tenets of critical care medicine is to ensure adequate tissue oxygenation. This determination is often based on the physical examination and the interpretation of standard hemodynamic parameters such as blood pressure, central venous pressure (CVP), and urine output. However, studies have demonstrated significant discordance between assessments based on these parameters and those based on measurements of cardiac function, cardiac output (CO), and tissue oxygenation (1,2,3,4,5,6,7,8). The pediatric intensivist must appreciate that no single observation or measurement taken in isolation can provide adequate hemodynamic monitoring. Rather, it is the application of a range of observations and measurements that allows the care team to balance oxygen delivery with the child’s metabolic demands. The type and intensity of hemodynamic monitoring should be calibrated to prevent tissue hypoxia episodes and, when necessary, to document that adequate tissue oxygenation has been restored.exhibit this pattern, the core-peripheral temperature gradient neither correlates well with other hemodynamic measurements nor predicts circulatory collapse (2,11).
preferred method in smaller children due to improved accuracy of volume infused, which should be taken into account when calculating fluid balance. Infusion sets should be renewed every 72 hours to minimize line infections.
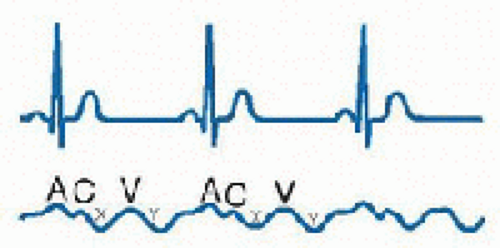
positive and two negative waves: the “a” wave follows the p wave on the ECG and is produced by atrial contraction; the “c” wave is produced by ventricular contraction and bulging of the tricuspid valve upward into the RA; the “x” descent results from atrial relaxation; the “v” wave results from right atrial filling and occurs in late systole before opening of the tricuspid valve; and the “y” descent results from opening of the tricuspid valve and passive filling of the right ventricle (RV). The CVP is the mean right atrial pressure, which approximates the right ventricular end-diastolic pressure, when right ventricular compliance and tricuspid valve function are normal. When ventricular function and compliance are diminished, atrial systole generates an end-diastolic pressure (reflected in the “a” wave) that is disproportionately higher than the mean pressure (14). Cannon “a” waves are produced when right atrial contraction occurs against a closed tricuspid valve, as in atrioventricular disassociation. Prominent “v” waves may be seen in tricuspid insufficiency and rapid “x” and “y” descents are suggestive of pericardial disease and restraint.
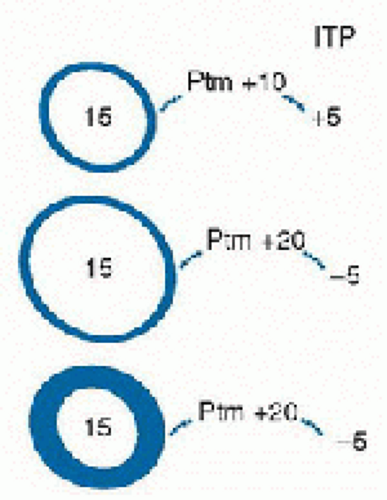
 Ventricular compliance is the ratio of the change of ventricular pressure (ΔP) to the change in ventricular volume (ΔV). One of the challenges of relying on the central venous (or ventricular filling) pressure as an indication of ventricular volume is that the relationship between volume and pressure (i.e., compliance) for a distensible chamber varies considerably from moment to moment in patients with cardiopulmonary disease (15). The effective compliance of the ventricle is affected by pericardial pressure and intrathoracic pressure. Thus, the end-diastolic transmural pressure (ventricular pressure-pericardial pressure) and ventricular compliance are the determinants of ventricular end-diastolic volume (Fig. 71.4). With a decrease in compliance, a greater distending pressure is needed to maintain ventricular filling. Ventricular compliance may be diminished as result of:
Ventricular compliance is the ratio of the change of ventricular pressure (ΔP) to the change in ventricular volume (ΔV). One of the challenges of relying on the central venous (or ventricular filling) pressure as an indication of ventricular volume is that the relationship between volume and pressure (i.e., compliance) for a distensible chamber varies considerably from moment to moment in patients with cardiopulmonary disease (15). The effective compliance of the ventricle is affected by pericardial pressure and intrathoracic pressure. Thus, the end-diastolic transmural pressure (ventricular pressure-pericardial pressure) and ventricular compliance are the determinants of ventricular end-diastolic volume (Fig. 71.4). With a decrease in compliance, a greater distending pressure is needed to maintain ventricular filling. Ventricular compliance may be diminished as result of:
myocardial disease (hypertrophic or ischemic myocardium);
elevated operating volumes, as occurs in systolic heart failure and elevated afterload;
pericardial disease;
an increase in intrathoracic pressure, as occurs with positive pressure ventilation, lower airway disease, and excessive lung volumes;
large pleural effusions.
pressures exceed right ventricular pressures. If the interventricular septum shifts from its normal position, either due to an increase in right or left ventricular diastolic pressure, the compliance and therefore pressure and volume (filling) of the contralateral ventricle is altered.
TABLE 71.1 COMMON HEMODYNAMIC VARIABLES | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree