KEY POINTS
The complications of hematopoietic stem cell transplantation generally relate to the consequences of the cytoreductive therapy, infections, and in the case of allogeneic transplants, immunosuppression and development of graft-versus-host disease.
Graft-versus-host disease remains one of the most important complications of allogeneic transplantation.
Stem cell transplant recipients may require admission to the intensive care unit for close monitoring for volume and electrolyte issues, vasopressor or renal support, and mechanical ventilation.
The approach to the diagnosis and management of infectious disorders in the stem cell transplant recipient is dependent on the underlying disease and prior therapy, timing of the infection relative to the transplant, the type of transplant, the patient’s immunologic history and comorbidities.
Pulmonary complications develop in up to 60% of allogeneic transplant recipients and are the immediate cause of death in approximately half of the cases.
Major noninfectious pulmonary complications in the early transplant period include idiopathic pneumonia syndrome, diffuse alveolar hemorrhage, and periengraftment respiratory distress syndrome; bronchiolitis obliterans syndrome and bronchiolitis obliterans organizing pneumonia occur later.
Despite advances in supportive care in the intensive care unit, the mortality rate of allogeneic transplant recipients who develop respiratory failure and multiple organ failure remains extremely high.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) has become an expanding modality for the treatment of benign and malignant hematologic diseases. While HSCT has been shown to be of benefit in only a few nonhematologic malignant diseases such as relapsed testicular cancer and neuroendocrine tumors, it has been studied in clinical trials in a variety of others such as renal cell and breast cancer, without major efficacy.1 In the area of benign diseases, it can restore hematopoiesis and/or immune function in congenital or acquired immune deficiency or marrow failure states. The most common diseases for which HSCT is performed are acute leukemia, myelodysplastic syndrome, Hodgkin and non-Hodgkin lymphomas, multiple myeloma, and less common disorders such as aplastic anemia.2 Classical HSCT is a lifesaving procedure which utilizes high doses of chemotherapy and/or radiotherapy. In the case of malignant disease, it is a treatment modality which is used after at least one and often many courses of standard chemotherapy. Greater numbers of patients over a wide age range are undergoing transplantation as a part of their oncologic therapy, and more of these patients are becoming survivors.3
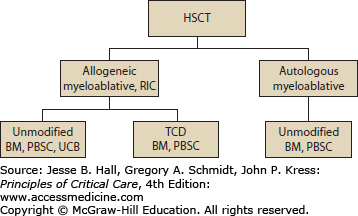
A stem cell transplant can be broken down into three components: the graft, the conditioning, and, in some types of transplant, the immunosuppression. There are two types of HSCT based on the source of the graft—“autologous”—when the stem cells are harvested from the patient at the time of blood count recovery after chemotherapy, or after receiving a white blood cell growth factor (granulocyte-colony stimulating factor [G-CSF]) or stem cell mobilizer (plerixafor)—which mobilizes bone marrow stem cells into the peripheral blood from which they can be collected by apheresis. The second type of stem cell transplant is “allogeneic” which utilizes stem cells donated from a family member, an unrelated donor, or umbilical cord blood (Fig. 94-1). When derived from a donor, the stem cells are matched to the patient using the antigenic determinants that mediate tissue graft rejection responses, primarily the human leukocyte antigens (HLAs). These are encoded by genes of the major histocompatibility complex located on chromosome 6.4 A fully HLA matched sibling is the preferred donor source because the risk of graft rejection and graft-versus-host disease (GVHD) is lowest with this source of cells. When a matched related sibling is not available, an unrelated fully matched donor is the preferred alternative.5 Unfortunately, due to a limited availability, sometimes only a partially matched or “mismatched” donor can be identified. Additional stem cell sources used in the allogeneic setting include umbilical cord blood and stem cells from a haploidentical family member. The stem cells can be obtained directly from the bone marrow by performing many aspirations from the posterior iliac crests under general anesthesia, by apheresis of stem cells mobilized from the bone marrow into the peripheral blood after administering G-CSF or stem cell mobilizer to the donor, or in the form of umbilical cord blood stem cells which were harvested from the placenta at the time of a birth. In an autologous transplant, the stem cells are only used to rescue the bone marrow from the damage caused by the chemotherapy, radiotherapy, and/or antibody therapy given just prior to the stem cell infusion to treat the malignancy. Stem cells in an allogeneic stem cell transplant rescue the bone marrow from treatment damage with cells that are free of disease. In addition, some of these stem cells, as well as the lymphoid cells that accompany the stem cells, develop into a new immune system, which may provide a biologic effect (allo effect) against the tumor referred to as the graft-versus-leukemia or graft-versus-tumor effect (GvT). ABO incompatibility between the patient and the donor requires manipulation of the graft prior to infusion with red cell or plasma depletion. A limited number of allogeneic transplant centers perform CD34+ selection of stem cells to remove many of the accessory cells (such as lymphocytes) prior to their infusion. Such manipulation of the graft may be referred to as CD34+ selection or T-cell depletion (TCD). The removal of T lymphocytes reduces the risk of GVHD, one of the major complications of allogeneic stem cell transplantation (see the section “Graft-Versus-Host Disease”).
The second component of a transplant is the “conditioning,” which is the treatment that prepares the patient for transplantation and generally incudes chemotherapy, radiotherapy, and/or antibody therapy. The conditioning is administered to treat any disease which may remain following standard chemotherapy used by the general oncologist to treat the disease. There are at least two forms of conditioning regimens. Myeloablative conditioning is the classical form. The intensity of this conditioning is such that the hematopoietic system would not be expected to recover, or would take a very prolonged time to recover, without being rescued by the infusion of the stem cells. The very prolonged period of pancytopenia, in such a setting, places the patient at high risk of life-threatening infections or bleeding and ultimately death. A newer type of transplant, developed over the past decade, utilizes nonmyeloablative conditioning. In this case, the chemotherapy and radiotherapy used in the conditioning is generally less intensive and is not expected to destroy the bone marrow, but rather may provide some treatment of the tumor, will make some space in the marrow for the new stem cells, and suppresses the patient’s immune system so that the stem cells and new immune system can grow. This nonmyeloablative type of transplant is used in large part for the immunologic effect of an allogeneic stem cell transplant. The intensity of this conditioning is milder than the myeloablative conditioning, and the patient’s marrow would be expected to recover even if the transplant failed. These regimens have caused less early posttransplant morbidity and mortality and have extended the age of eligibility for allogeneic HSCT to patients in their seventies, and to patients with medical comorbidities that previously would have precluded them from a transplant.
The third component of the allogeneic stem cell transplant is the immunosuppression (this treatment is not needed for an autologous transplant because the patient would be receiving their own cells back). Since the allogeneic transplant patient is receiving both new bone marrow stem cells and cells to generate a new immune system, the latter cells from the donor must be kept under control with immunosuppressive drugs until they become acclimated to living in the patient.
In summary, HSCT involves the use of chemotherapy, radiotherapy, or biologic therapy known as the conditioning, followed by infusion of stem cells to (a) rescue the bone marrow from the consequences of the therapy and (b) in the case of allogeneic HSCT, to provide a new immune system and hence, hopefully, a biologic effect against any residual disease. The most potentially complicated transplant is the myeloablative allogeneic HSCT. The complications of HSCT generally relate to consequences of the cytoreductive therapy (the conditioning), infections, and in the case of allogeneic HSCT, immunosuppression and development of GVHD (see the section “Graft-Versus-Host Disease”). Certain types of transplants can be expected to result in more complications than others—these include allogeneic HSCTs which use mismatched volunteer donors or cord blood as the source of the graft, patients with relapsed/refractory disease at the time of transplant, and patients with end-organ dysfunction pretransplant.
Although HSCT can be lifesaving, the vulnerable condition of the patient generated by the conditioning and/or immunosuppression can also make it a “life-threatening” procedure. Furthermore, a proportion of these patients will require transfer to the intensive care unit (ICU) for more advanced level of care than can be provided on a bone marrow transplant ward. The reasons for ICU admission of HSCT recipients are shown in Table 94-1. ICU-directed care may include close monitoring for volume and electrolyte issues, vasopressor support, hemodialysis, and mechanical ventilation. The reported rates of ICU admission for autologous and allogeneic HSCT recipients have ranged from 5% to approximately 60%.6-14 Although HSCT patients may require ICU care at any time during their transplant course, the highest incidence is in the peritransplant period. ICU admission beyond the first month posttransplant is generally related to infection but may also relate to the long-term complications of transplantation.
Reasons for Intensive Care Unit Admission of HSCT Recipients
|
COMPLICATIONS OF THE CONDITIONING REGIMEN
The conditioning prepares the patient for the transplant. The agents used most frequently in conditioning for HSCT are listed in Table 94-2, along with their major toxicities. Most are alkylating agents with their major toxicity being myelosuppression. As would be expected drug dosing does impact on the severity of these toxicities. Sites of greatest effect are the bone marrow resulting in myelosuppression and peripheral blood cytopenias, and the oropharynx and gastrointestinal (GI) tract manifested as oral and intestinal mucositis, pain, and diarrhea. The direct toxic effects to mucosa are most often caused by the alkylating agents. Side effects on other organs are less common and vary between agents.
Drugs Used in Conditioninga
| Busulfan—alkylating agent | Hepatotoxicity, mucositis, seizures, darkening of the skin, cataracts |
| Carmustine—alkylating agent | Infusion pain and burning of the face, hepatotoxicity, nausea, vomiting, hypotension, pulmonary fibrosis |
| Cyclophosphamide—alkylating agent | Electrolyte imbalances, cardiomyopathy, SIADH, nausea, vomiting, diarrhea, hemorrhagic cystitis |
| Cytosine arabinoside—antimetabolite | Nausea, vomiting, diarrhea, neurotoxicity, ocular irritation, capillary leak syndrome, hand-foot syndrome |
| Etoposide—topoisomerase II inhibitor | Infusion hypotension/hypertension, acidosis during infusion, nausea, vomiting, diarrhea, rash, mucositis |
| Fludarabine—antimetabolite | Nausea, vomiting, fever, immunosuppression |
| Ifosfamide—alkylating agent | Nephrotoxicity, neurotoxicity, acidosis during infusion |
| Melphalan—alkylating agent | Cardiac, nausea, vomiting, diarrhea, mucositis, renal dysfunction |
| Thiotepa—alkylating agent | Hand-foot syndrome, nausea, vomiting, diarrhea, mucositis, mental status changes, and psychosis |
| Rituximab—monoclonal antibody against CD19 | Infusion reactions, suppression of B-cell function and hypogammaglobulinemia, cytopenias, reactivation of hepatitis B and other viral infection, progressive multifocal leukoencephalopathy, mucocutaneous reactions |
| Immunosuppressive drugs | |
| Alemtuzumab | Anaphylactic reaction, hypotension, fever, rigors, reactivation, and susceptibility to viral infections |
| Antithymocyte globulin | Anaphylactic or infusion reactions, hypotension, fever, rigors, rash, reactivation of viral infections |
| Cyclosporine | Nephrotoxic, hypertension, neurotoxicity, immunosuppressive |
| Mycophenolate mofetil | Nausea, vomiting, diarrhea, bone marrow suppression, immunosuppressive |
| Sirolimus | Hyperlipidemia, cytopenias, thrombotic microangiopathy, hepatotoxicity, pulmonary toxicity, immunosuppressive |
| Tacrolimus | Nephrotoxic, hypertension, neurotoxicity, immunosuppressive |
The dose of total body irradiation varies widely when used as conditioning for HSCT. Fractionating the doses tends to reduce the toxicities. Doses less than 900 cGy are considered nonmyeloablative but as the dose is escalated, the incidence of GI complications such as nausea, vomiting, diarrhea, and mucositis will increase. Furthermore, the higher the total dose of radiation used, the more significant the impact on the bone marrow and the peripheral blood counts. In the nonmyeloablative transplants, often only one dose of total body irradiation (200 cGy) is administered for its immunosuppressive effect. This dose produces little in the way of systemic side effects except possibly some nausea.
The marrow suppression produced by high doses of chemotherapy and radiotherapy used for myeloablative HSCT results in peripheral blood cytopenias, increasing the risk of infection, bleeding, and the need for transfusions. The development of mucositis and the loss of normal barriers to pathogens in the oral cavity and GI tract compound the risk of infection generated by the cytopenias alone. If oral nutrition cannot be maintained, patients will need total parental nutrition (TPN) with its inherent risks for infection. Often narcotic analgesics are needed for pain management—compounding the issues of nausea and producing somnolence. In addition to their marrow suppressive effects, many of the agents used for conditioning in the allogeneic transplant setting are also lymphocytotoxic—producing immunosuppression, and thus expanding the spectrum of pathogens to which the patient is susceptible (see the section “Infectious Complications”).
In the case of autologous HSCT, the conditioning includes only the high-dose chemotherapy, radiotherapy, and/or monoclonal antibody therapy, and does not require immunosuppression. As a consequence, the complications are due to the direct toxic effect of this therapy and the resulting cytopenias.
Additional agents that may be used during the conditioning in allogeneic HSCT include antithymocyte globulin and immunosuppressive medications used to prevent GVHD and graft rejection (Table 94-2). The primary activity of these agents is lymphocytotoxic—suppressing both the patient’s immune system to prevent rejection of the transplanted graft and the transplanted immune system provided by the donor. This immunosuppressive effect results in the patient becoming more susceptible to opportunistic pathogens such as viruses and Pneumocystis jiroveci (see the section “Infectious Complications”).
GRAFT-VERSUS-HOST DISEASE
Graft-versus-host disease (GVHD) remains the most important complication of allogeneic HSCT. This clinicopathologic syndrome is an immunologic reaction of donor lymphocytes to “foreign” antigens present on the surface of host cells. The most important proteins are HLAs encoded by the major histocompatibility complex but differences in “minor” antigens, not currently tested in donor selection, are targets for both GVHD and graft-versus-leukemia. The requirements for a diagnosis of GVHD defined by Billingham 50 years ago remain valid today: (1) the graft must contain immunocompetent cells; (2) the host must express tissue antigens that are not present in the transplant donor, and (3) the host must be incapable of mounting an effective response to eliminate the transplanted cells.15 While historically the classification of GVHD was based on timing (acute—occurring within 100 days after HSCT and chronic—occurring after 100 days), the current consensus is that this distinction be made by clinical manifestations rather than time from transplantation alone.16 In addition to acute and chronic GVHD, the National Institutes of Health (NIH) classification includes late-onset acute GVHD (after day 100) and an overlap syndrome with features of both acute and chronic diseases.17
Although histocompatibility matching is critical in limiting the incidence and severity of acute GVHD, this alone cannot prevent it. Almost all recipients of unmodified allogeneic HSCT develop GVHD if not given posttransplant immunosuppression. The primary pharmacological strategy to prevent GVHD is inhibition of calcineurin, a cytoplasmic enzyme important for activation of T cells. The calcineurin inhibitors, cyclosporin and tacrolimus, have similar mechanisms of action, clinical effectiveness, and toxicity. The most serious side effects of calcineurin inhibitors include renal insufficiency, thrombotic microangiopathy manifesting as severe cytopenias, and posterior reversible encephalopathy syndrome presenting with seizures. Calcineurin inhibitors are usually administered in combination with other agents such as methotrexate or less toxic, mycophenolate mofetil.18 Sirolimus is an immunosuppressive agent that is structurally similar to tacrolimus but is not a calcineurin inhibitor. It has been used in combination with tacrolimus.19,20 In addition to pharmacologic approaches, removal of donor T lymphocytes from the stem cell inoculum either in vitro or in vivo has been effective in preventing GVHD. Currently, strategies of T-cell depletion include negative selection of T cells ex vivo, positive selection of CD34+stem cells ex vivo, and use of antibodies against T cells in vivo and ex vivo.21
Incidence: Overall, the incidence of moderate-to-severe acute GVHD with HLA identical siblings ranges from <10% to 60% depending on prophylaxis and risk factors.21 The frequency of acute GVHD is directly related to the degree of mismatch between the patient and donor HLA proteins. The incidence increases with patient age and degree of HLA disparity between the host and the donor. Other contributing factors are the use of an unrelated donor, the number of T cells transplanted, the sex mismatching and donor parity, the severity of tissue injury during cytoreduction, and the type of prophylaxis used.
Pathophysiology: Although the principal effector cells responsible for GVHD are the immunocompetent donor cytotoxic T lymphocytes, the first step of acute GVHD entails the activation of antigen presenting cells (APCs) in the host.22 Host tissues, damaged by the underlying disease, previous infections, as well as the transplant conditioning regimen itself, respond by producing “danger signals” including proinflammatory cytokines and costimulatory molecules, resulting in APC activation.23 The second step follows APC activation and includes the proliferation and activation of donor T cells. Activation of donor immune cells results in rapid biochemical cascades that induce transcription of genes for many cytokines and their receptors (interferon-γ, interleukin-2, and TNF-α). These molecules work synergistically with donor cells in producing the third effector step of GVHD, thereby amplifying local tissue injury and promoting target tissue destruction.
Clinical Features: The hallmarks of acute GVHD at onset include involvement of the skin (81% of patients), gastrointestinal tract (54%), and liver (50%).24 Skin involvement is the most frequent and earliest manifestation of acute GVHD. The typical presentation of skin GVHD is a pruritic, maculopapular rash that can spread throughout the body but spares the scalp. Severe cases can cause diffuse blistering and ulceration. Gastrointestinal-tract involvement usually presents with large volume (>2 L/day) diarrhea but can also include vomiting, anorexia, abdominal pain, or bleeding. Hepatic GVHD often manifests with jaundice, cholestasis, and to a lesser degree transaminitis. It can be difficult to distinguish GVHD from other causes of hepatic dysfunction and a biopsy may be helpful for diagnosis. The severity of GVHD is commonly determined by staging the extent of involvement of the three main target organs described (skin, GI tract, and liver). Historically, the most commonly used grading system was the Glucksberg grading system first published in 1974 then revised by Thomas et al in 1975.25-27 This system formulated a grade based on stage of severity (0-4) of the skin, liver, and GI tract using both objective and subjective assessment of performance status. These stages are then combined to calculate an overall grade, 0-IV. A revised system was developed by the International Bone Marrow Transplant Registry (IBMTR) in 1997 that retained the objective criteria but eliminated the subjective component in an effort to simplify grading and allow for comparison between transplant centers. A clinical grading system (0-IV) is based on highest stage of any single organ system involved (Table 94-3).27 Severity is described as Grade I (mild) to Grade IV (severe).28 Severe GVHD has a poor prognosis with 25% long-term survival (5 years) for grade III disease and 5% for grade IV disease.29
Staging of Acute GVHD
| Stage | Skin | Liver | GI tract |
|---|---|---|---|
| 0 | No rash due to GVHD | Bilirubin <2 mg/dL | None (<280 mL/m2) |
| I | Maculopapular rash <25% of body surface area without associated symptoms | Bilirubin from 2 to <3 mg/dL | Diarrhea >500-1000 mL/day; nausea and emesis |
| II | Maculopapular rash or erythema with pruritus or other associated symptoms ≥25% of body surface area or localized desquamation | Bilirubin from 3 to <6 mg/dL | Diarrhea >1000-1500 mL/day; nausea and emesis |
| III | Generalized erythroderma; symptomatic macular, papular, or vesicular eruption with bullous formation or desquamation covering ≥50% of body surface area | Bilirubin 6-15 mg/dL | Diarrhea >1500 mL/day |
| IV | Generalized exfoliative dermatitis or bullous eruption | Bilirubin >15 mg/dL | Diarrhea >1500 mL/day; abdominal pain or ileus |
Diagnosis and Management: The diagnosis of acute GVHD is rarely straightforward. Dermatitis, jaundice, and enteritis in the transplant setting have a variety of potential etiologies including but not limited to GVHD. Toxicity from chemoradiotherapy, supportive medications, or infection must be considered, as well as transplant-specific complications such as hepatic venoocclusive disease. Biopsies can be helpful but often fail to provide a definitive diagnosis. Furthermore, a diagnosis supported by clinical presentation and even biopsy does not eliminate the possibility of coexisting conditions. Repeat blood cultures and histologic evaluation for organ invasive viral or other opportunistic infections should be prompted by persistent fever and symptoms. In addition, signs and symptoms in any organ not responding to treatment for presumed acute GVHD should trigger further evaluation.
Treatment for established acute GVHD necessitates additional immunosuppressive therapy. First-line treatment is generally corticosteroids.23 With mild to moderate involvement of skin and upper GI tract, topical formulations may be adequate, steroid creams for skin and oral budesonide for upper GI tract. Systemic corticosteroids are the standard treatment if there is lack of response to local steroids, or for treatment of hepatic or lower GI involvement. No randomized trials have established a regimen superior to corticosteroids as primary treatment for acute GVHD nor has any study determined the most efficacious dose.30 The conventional starting dose is methylprednisolone 1 to 2 mg/kg daily with a taper of approximately 10% per week once symptoms improve although this approach is highly personalized. Unfortunately, complete remission rates from systemic steroids are less than 50%, and more severe GVHD is less likely to respond to treatment.31 The survival rates for steroid-resistant GVHD are quite poor, and death is most often due to infection.
There is no standard of care for second-, third-, or fourth-line therapy for acute GVHD in the setting of steroid resistance and disease progression. Agents that have been used include calcineurin inhibitors, sirolimus or mycophenolate (if not used for prophylaxis), antithymocyte globulin, daclizumab, infliximab, etanercept, ontak, and pentostatin. Mesenchymal stem cell infusions are a more recent approach that has shown promising results in clinical trials, especially in patients with GI involvement.32,33
Immunosuppression in patients undergoing treatment for acute GVHD can be profound and continued surveillance for viral, bacterial, or fungal infection is obligatory. Prophylactic broad-spectrum antibiotics should be used during periods of neutropenia and antibiotic coverage for encapsulated organisms at a minimum should be provided once neutrophil counts have recovered. Any fever should be aggressively evaluated with strong consideration given to adjustment of antibiotics, especially in the absence of intact mucosal and skin barriers. Adequate nutrition, hydration, and electrolyte balance are vital but often difficult to maintain with severe enteric GVHD. Malabsorption is common and oral nutrition and electrolyte support is often not practical. Careful monitoring of diarrhea volume and electrolyte levels should be maintained and prolonged periods of total parenteral nutrition may be required. Cramping and diarrhea may necessitate antidiarrhea medications and use of parenteral opiate analgesics, even at the risk of an ileus. In severe enteric GVHD, bleeding from the GI tract will require frequent transfusion support. Bleeding in the setting of decreasing diarrhea volume should trigger evaluation for the site(s) of severe or persistent bleeding with endoscopic or radiologic procedures. Severe involvement of the skin resulting in desquamation requires aggressive volume repletion, intense nursing care and consultation with dermatologists, and plastic surgeons or burn specialists. Depressed peripheral blood counts and poor marrow function may be manifestations of severe acute GVHD. Thrombocytopenia due to decreased platelet production may be compounded by rapid turnover in the setting of fever and hemorrhage. Maintenance of hemostasis often requires vitamin K supplementation, transfusions of platelets, and in the setting of hepatic failure, the administration of fresh frozen plasma.
Definition: Chronic GVHD is a heterogenous, alloimmune syndrome. It is the major cause of late nonrelapse mortality after HSCT.34,35 Advanced age of the recipient, use of peripheral blood stem cells versus bone marrow stem cells, and a history of acute GVHD are significant risk factors for chronic disease; however, acute GVHD does not necessarily evolve into chronic disease and chronic GVHD can occur in the absence of prior acute disease.
Immunology: The pathophysiology and immunobiology of chronic GVHD remain undefined. Several different hypotheses regarding the pathogenesis of chronic GVHD have emerged from animal studies including thymic damage caused by acute GVHD, resulting in failure of T-cell selection, cytokine mediators that may propagate autoimmune-like tissue injury, donor B cells and antibody mediated mechanisms, as well as insufficiency of T regulatory cells.34
Manifestations: Clinical symptoms of chronic GVHD may involve the skin, nails, mucous membranes, GI tract, and liver. The characteristic pigmentation and sclerosis of the skin, lichenoid oral plaques, esophagitis, polyserositis, and oral and ophthalmic sicca syndrome resemble the manifestations of autoimmune collagen-vascular disorders. GI involvement is often manifested by anorexia, weight loss, and esophageal strictures. Hepatic involvement presents with cholestasis and jaundice but can also present with transaminitis. Chronic pulmonary diseases are seen in 10% to 20% of patients with chronic GVHD and are divided between diffuse pneumonias and bronchiolitis obliterans (see the section “Bronchiolitis Obliterans Syndrome”). The diagnosis of chronic GVHD requires at least one diagnostic sign that is found only in chronic GVHD or at least one distinctive sign highly suggestive of chronic GVHD.36 A biopsy may be helpful in ruling out other potential diagnoses such as infection or drug effect. The NIH consensus has recommended a system of scoring chronic GVHD manifestations in eight sites on a 0-3 scale. This system was not designed to predict mortality but to assess the severity and functional impact of chronic GVHD. It replaces the historical categories of limited and extensive involvement. Mortality from chronic GVHD tends to be lower when the disease is limited to the skin and liver and when it occurs in the absence of prior acute GVHD. Death is attributed to GVHD even if it is due to a complicating infection as a result of the immunosuppressive medications needed to treat the GVHD.
Diagnosis and Management: The diagnosis of chronic GVHD is most often made in the outpatient setting and relies on clinical assessment, laboratory, biopsy, and radiologic evaluation similar to that outlined for acute GVHD. When managing patients with chronic GVHD, it is important to remember that although chronic GVHD may be a significant contributor to late transplant morbidity and mortality, it may also be associated with an important therapeutic graft-versus-tumor effect. Because our understanding regarding the biology of chronic GVHD is incomplete, current treatment strategies are based on nonspecific, global immunosuppression. Use of corticosteroids (with or without a calcineurin inhibitor) is a standard first-line approach. Multiple second-line treatments have been studied but none have achieved widespread acceptance. Generally, treatments involve the addition of many of the same drugs as are used for treatment of acute GVHD. A few combinations such as tacrolimus and MMF have demonstrated more consistent benefit than others. Extracorporeal photophoresis has shown some promise in select patients with dermatologic involvement.37 Ancillary therapy and supportive care to prevent infections, optimize nutrition, ameliorate morbidity, and optimize functional performance and capacity are critical components of the management of chronic GVHD. Treatment is often complicated by infection. All patients with chronic GVHD are at risk for infection with encapsulated organisms, particularly Streptococcus pneumoniae but also H influenzae and Neisseria meningitides. Prophylactic antibiotics should be given to all patients with chronic GVHD receiving systemic immunosuppressive treatment.38 Patients with chronic GVHD are at risk for the development of overwhelming sepsis and involvement of the critical care physician in such settings is particularly helpful.
INFECTIOUS COMPLICATIONS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree