Health Maintenance and the Role of Screening
Public interest in health maintenance or, more positively, health enhancement varies over time and in different segments of the population. Some Americans have demonstrated their interest in exercise, good dietary habits, maintenance of appropriate body weight, and stress reduction. Despite reliable evidence and public acceptance of these associations between elements of lifestyle and health, however, many people continue to indulge in self-destructive habits such as smoking, overeating, and alcohol abuse. For instance, in recent years, we have seen a dramatic increase in the prevalence of obesity and the incidence of type 2 diabetes. Efforts to alter such behavior are often frustratingly ineffective. Patients who seek reassurance from physician visits that include routine screening procedures often persist in behavior that greatly increases their risk of morbidity.
Physicians must acknowledge their primary role in prevention as the leader of a team that can effectively engage, educate, and motivate patients to live healthier lives. Accurate information regarding risk factors is most likely to reinforce health-enhancing behavior and alter self-destructive behavior. Clinicians must appreciate the potential for behavior modification and familiarize themselves with local resources that can help patients to identify and overcome barriers to healthy behavior. Routine screening for specific diseases—the health maintenance activity most closely identified with the physician—should be performed selectively. The limits of screening tests, as well as their potential health benefits, should be clearly understood by every primary care physician.
Specific risk factors and screening tests are discussed in subsequent chapters. This chapter focuses on the following question: What makes a disease or risk factor worth screening for? The relationship between prevalence and the predictive value of a test is particularly important in the screening situation (see Chapter 2). Because the physician should be more interested in improving health outcomes for patients than in simply providing them with diagnoses, elements of the natural history of the disease and of the effectiveness of therapy are critically important.
Whether a screening policy results in improved health outcomes depends on the characteristics of the disease(s), the test(s), and the patient population. These are summarized in Table 3-1.
Screening tests are performed to identify asymptomatic disease. The alternative is to wait until the patient presents with symptoms and then make a diagnosis. The question then is: What makes a disease worth diagnosing early? The practical objective of screening is prevention of morbidity and mortality, not simply early diagnosis. There is little benefit to the patient, and perhaps considerable harm, in advancing the time of diagnosis of a disease for which earlier treatment does not influence outcome.
TABLE 3-1 Criteria for Screening | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
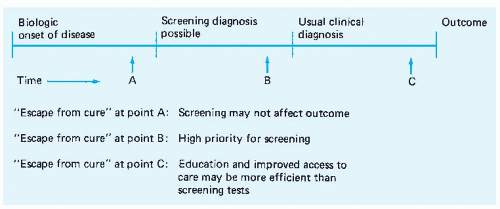
The importance of the natural history of the disease and the effectiveness of therapy can be illustrated by considering Figure 3-1. As it shows schematically, some variable time after the biologic onset of a disease, a diagnosis is possible with the use of a screening test. This is followed by another variable time period during which the patient has no symptoms. Usually, a short time after symptoms appear, the clinical diagnosis is made. Eventually, after the course of therapy has been selected and completed, there is an identifiable clinical outcome that can range from cure and complete health to death.
Often, outcome depends somewhat on the point during the natural history of the disease at which therapy is initiated. This is clearest in the case of localized versus metastatic cancer. Many tumors can be readily excised, and the patient cured of the disease, during early stages. The opportunity for cure is often lost when tumor spread makes excision or other local therapy impractical. The “escape from cure” may not be as dramatic as the point of tumor metastasis; a disease may simply become more refractory to therapy, which increases the likelihood of morbid complications. The practical purpose of screening is to advance the time of the diagnosis to a point in the natural history of the disease when a relative or absolute “escape from cure” is less likely to have occurred.
Although the natural history of any disease varies a great deal among persons afflicted, some generalizations are worthwhile. If an “escape from cure” generally occurs at point A in Figure 3-1 or at any point before available screening tests can detect the disease in question, the value of screening must be questioned. The most common result will be bad news sooner for the patient but no difference in outcome. If “escape from cure” routinely occurs after symptoms appear (e.g., at point C), screening may be valuable but can likely be supplanted by patient and professional education programs aimed at ensuring early presentation and prompt diagnosis. Diseases in which “escape from cure” generally occurs after the disease is detectable but while it remains asymptomatic (e.g., at point B) are the most appropriate targets of screening efforts.
Several points about the evaluation of screening programs can also be made with reference to Figure 3-1. Critics of indiscriminate screening point out that the benefits of a screening program can easily be overestimated if the relationship between time of diagnosis and natural history is not understood. One fallacy results from neglecting the importance of lead time when evaluating the effect of screening on subsequent survival. Because screening has the potential to advance the time of diagnosis from one point in the natural history to another and because survival is, by necessity, measured from the time of diagnosis rather than from the time of onset, the survival of patients whose diseases are detected by screening should be expected to be longer than that of patients who present symptomatically. Extensive follow-up data on many patients allow approximation of the average length of time by which the diagnosis is advanced by screening. This illusory gain in survival—the lead time—can then be subtracted from any measured difference in survival duration to learn the true benefits of the screening program.
The second fallacy that can lead to overestimation of screening benefits depends on the variability in natural history among individual cases of the same disease. Patients who have lessaggressive disease and so spend more time in a detectable but asymptomatic stage are, other things being equal, more likely to have their disease detected by a screening test than are patients with more aggressive disease. If patients with indolent, asymptomatic disease are more likely to have an indolent clinical course after diagnosis, patients with disease diagnosed by screening should be expected to have longer survival rates than patients who present symptomatically. Arguments about the effect of such biologic determinism versus that of advancing the time of diagnosis have most frequently been raised with regard to breast and prostate cancers, but they apply generally to all screening. This potential bias toward prolonged survival among patients with disease detected by screening tests has been called time-linked bias sampling. An extreme form of this phenomenon is overdiagnosis.
Overdiagnosis occurs when disease that would not have been diagnosed during the patient’s lifetime is diagnosed because a screening test was performed. This is the case for most prostate cancers detected by screening and for approximately half of all cases of ductal carcinoma in situ of the breast. When overdiagnosis due to screening causes a surge in the incidence of diagnosed disease, as occurred after the introduction of prostate-specific antigen screening for prostate cancer, demand for screening among patients tends to increase because seeing friends and relatives with the disease makes them feel vulnerable. This has been termed the screening paradox. Increased recognition of the incidence of overdiagnosis of breast cancer and the subsequent overtreatment of women who never would have been diagnosed during their lifetimes led to a change in screening policy in the English National Health Service in 2012. Women are informed of the potential harms as well as benefits before screening.
None of these arguments is meant to deny the value of screening for conditions that can be detected at a time when they are more easily or effectively treated. They simply advise caution in interpreting apparently favorable results based on unsophisticated measures of effectiveness.
Diseases worth identifying usually have a relatively low prevalence in the asymptomatic population. As a result, the specificity of the diagnostic test used is the principal determinant of the predictive value positive of the test. Tests that may be very useful in diagnosis when the prior probability of disease is 10% or
20% may produce an unacceptable number of false-positive results when used in a screening situation. Such nonspecificity has been referred to as the cost of a screening test. The costs, including morbidity and patient concern, of diagnostic evaluations among patients with false-positive screening results can far outweigh other costs of a screening program. The sensitivity and the specificity of the screening test, costs, and patient acceptability are critical considerations in the decision to screen for disease. The importance of disease prevalence in determining the predictive value positive is one basis for the use of risk factors in screening policy. By limiting screening to a high-risk population, the physician in effect increases the prevalence of the disease in the population tested (and increases the prior probability of the disease in any individual patient), thereby increasing the predictive value positive and decreasing the false-alarm rate and the number of false-positive results.
20% may produce an unacceptable number of false-positive results when used in a screening situation. Such nonspecificity has been referred to as the cost of a screening test. The costs, including morbidity and patient concern, of diagnostic evaluations among patients with false-positive screening results can far outweigh other costs of a screening program. The sensitivity and the specificity of the screening test, costs, and patient acceptability are critical considerations in the decision to screen for disease. The importance of disease prevalence in determining the predictive value positive is one basis for the use of risk factors in screening policy. By limiting screening to a high-risk population, the physician in effect increases the prevalence of the disease in the population tested (and increases the prior probability of the disease in any individual patient), thereby increasing the predictive value positive and decreasing the false-alarm rate and the number of false-positive results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree