CHAPTER 10 HAEMATOLOGICAL PROBLEMS
ANAEMIA IN THE CRITICALLY ILL
Anaemia is common in critically ill patients and often necessitates repeated blood transfusion. Anaemia may be the direct result of an underlying disease process, but more commonly is multifactorial. Common factors that may contribute to anaemia are listed in Box 10.1.
Box 10.1 Factors contributing to anaemia in the critically ill
Blood loss associated with repeated surgical procedures
Anaemias of chronic disease (e.g. chronic renal failure)
Effects of underlying disease process
Haemolysis (e.g. drugs, antibodies, infection)
INDICATIONS FOR BLOOD TRANSFUSION
 Oxygen carriage and delivery. The oxygen content of blood is given by Hb × SaO2 × 1.34. Raising haemoglobin is an effective way of improving oxygen content and delivery. (See Oxygen delivery and oxygen consumption, p. 68.)
Oxygen carriage and delivery. The oxygen content of blood is given by Hb × SaO2 × 1.34. Raising haemoglobin is an effective way of improving oxygen content and delivery. (See Oxygen delivery and oxygen consumption, p. 68.) Myocardial function. Myocardial ischaemia and diastolic dysfunction may occur in the stressed heart when the haematocrit falls below 0.18. In the presence of coronary artery disease, the threshold is 0.24 or higher.
Myocardial function. Myocardial ischaemia and diastolic dysfunction may occur in the stressed heart when the haematocrit falls below 0.18. In the presence of coronary artery disease, the threshold is 0.24 or higher. Rheology. In vitro and probably in vivo, blood viscosity is reduced as haematocrit falls below 0.24 and rises above 0.3. This may have implications for perfusion of the microcirculation, particularly in critical illness and following vascular surgery. Recent evidence suggests that both very low and very high haematocrits are associated with impaired tissue perfusion. Haematocrit can be estimated from Hb (g / dL) × 3/100.
Rheology. In vitro and probably in vivo, blood viscosity is reduced as haematocrit falls below 0.24 and rises above 0.3. This may have implications for perfusion of the microcirculation, particularly in critical illness and following vascular surgery. Recent evidence suggests that both very low and very high haematocrits are associated with impaired tissue perfusion. Haematocrit can be estimated from Hb (g / dL) × 3/100. Immunology. There is some evidence that transfusion induces a degree of immunosuppression, particularly massive transfusion. This is important following any major surgery, and especially so where surgery has been performed for malignant disease.
Immunology. There is some evidence that transfusion induces a degree of immunosuppression, particularly massive transfusion. This is important following any major surgery, and especially so where surgery has been performed for malignant disease.BLOOD PRODUCTS IN THE UK
Recently, potential transmission of new variant Creutzfeldt–Jakob disease (vCJD) has become a concern. It is likely that in the near future screening of donors for vCJD will become available. Currently in the UK all blood products have white cells removed (leucodepletion) as a precaution against vCJD transmission (white cell count < 5 × 106). Continuing concerns regarding the potential for carriage and transmission of vCJD by the UK blood donor pool has resulted in some plasma products being sourced from outside the UK, principally from the USA.
The following component blood products are available.
Red cells
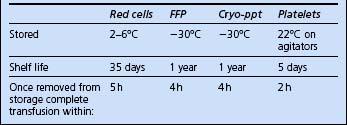
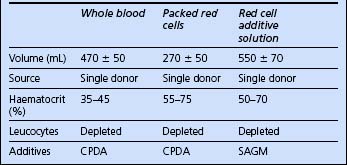
The blood products potentially available for red cell replacement are shown in Table 10.1.
Fresh frozen plasma
Recent concerns regarding virus transmission have resulted in treated plasma products becoming available. There are currently two: methylene blue treated and solvent detergent treated. The characteristics of available plasma products are compared in Table 10.2.
Cryoprecipitate
Cryoprecipitate is provided as one to six single donations per pack, suspended in 10–20 mL plasma. It contains fibrinogen and factor VIII. It is used to correct coagulopathy where fibrinogen levels are depleted. Six units generally raise fibrinogen levels by approximately 1 g/L.
ADMINISTRATION OF BLOOD PRODUCTS
Requesting blood products
 Ensure blood samples are carefully labelled and that the accompanying request forms are accurately completed.
Ensure blood samples are carefully labelled and that the accompanying request forms are accurately completed.Check recipient identity
 When possible, ask the patient to confirm his or her identity and that the details on the identification band are correct.
When possible, ask the patient to confirm his or her identity and that the details on the identification band are correct. Ensure that the patient’s name and identification number on the wristband match those on the intended blood product.
Ensure that the patient’s name and identification number on the wristband match those on the intended blood product.MAJOR HAEMORRHAGE
 Ensure adequate vascular access: at least 2 × 14-gauge peripheral lines or single large-bore cannula such as 8.5-Fr introducer sheath. This need not necessarily be inserted into a central vein; a peripheral vein may well be easier to cannulate in an emergency, and just as adequate.
Ensure adequate vascular access: at least 2 × 14-gauge peripheral lines or single large-bore cannula such as 8.5-Fr introducer sheath. This need not necessarily be inserted into a central vein; a peripheral vein may well be easier to cannulate in an emergency, and just as adequate. Continue background or maintenance fluids to provide free water, glucose and electrolyte requirements.
Continue background or maintenance fluids to provide free water, glucose and electrolyte requirements. Commence initial volume replacement with a crystalloid or simple colloid such as modified gelatin (e.g. Gelofusine or Haemaccel).
Commence initial volume replacement with a crystalloid or simple colloid such as modified gelatin (e.g. Gelofusine or Haemaccel). After 5 units of blood, consider changing to whole blood if available and / or giving FFP to minimize the effects of dilutional coagulopathy.
After 5 units of blood, consider changing to whole blood if available and / or giving FFP to minimize the effects of dilutional coagulopathy. Ensure adequate treatment of coagulopathy. In particular, keep the ionized calcium above 0.85 mmol / L. Maintain normothermia with active warming of the patient if necessary.
Ensure adequate treatment of coagulopathy. In particular, keep the ionized calcium above 0.85 mmol / L. Maintain normothermia with active warming of the patient if necessary. After 10 units, recheck clotting. Consider cryoprecipitate, platelets and further FFP (see Coagulopathy below).
After 10 units, recheck clotting. Consider cryoprecipitate, platelets and further FFP (see Coagulopathy below).RISKS AND COMPLICATIONS OF BLOOD TRANSFUSION
Complications of blood transfusion include fluid overload, hypothermia, hypocalcaemia, acidosis and dilutional coagulopathy. ARDS and multiple organ failure are also considered to be complications of massive transfusion. Bacterial contamination of blood occurs rarely and is usually fatal (platelet transfusion carries the greatest risk because of the need to store at room temperature).
Acute transfusion reactions are relatively uncommon. They include:







 In some hospitals, a form accompanies blood products, identifying the units issued; this is intended as a record to be placed in the patient’s notes. These forms are not intended to be used as part of the checking procedure, and do not help to ensure that the correct unit of blood is given to the correct patient. The only acceptable checking process is to confirm that the patient details on the product label and those on the patient’s wrist band are the same.
In some hospitals, a form accompanies blood products, identifying the units issued; this is intended as a record to be placed in the patient’s notes. These forms are not intended to be used as part of the checking procedure, and do not help to ensure that the correct unit of blood is given to the correct patient. The only acceptable checking process is to confirm that the patient details on the product label and those on the patient’s wrist band are the same.