Key Clinical Questions
Introduction
Admissions with diabetic ketoacidosis (DKA) as the primary diagnosis have increased over the past 30 years, with 120,000 recorded in the United States in 2005. However, mortality has been reduced to less than 5%. Hyperosmolar hyperglycemic syndrome (HHS) is less common, but it is associated with a mortality of up to 11% because of the greater age and comorbid conditions of the at-risk patient population.
Diabetic Ketoacidosis
DKA arises in patients with absolute or severe insulin deficiency. The resulting hyperglycemia combined with an increase in counter-regulatory hormones, including glucagon, catecholamines, cortisol, and growth hormone, precipitate proteolysis and lipolysis. Lipolysis causes the accumulation of acidic ketones, and hyperglycemia leads to osmotic diuresis and electrolyte loss, precipitating the characteristic metabolic acidosis and severe dehydration.
Symptoms of DKA generally develop over a short period of time and include polyuria, polydipsia, and weight loss. Abdominal pain and vomiting are common with acidosis, and must be distinguished from an acute abdomen. Decreased mentation and deep, labored Kussmaul respirations are advanced findings. Symptoms and signs of intercurrent illness should be sought. Leukocytosis and hyperamylasemia are often detectable at presentation and normalize with treatment.
A recent consensus statement has clarified diagnostic criteria for DKA (Table 148-1). Five laboratory conditions are typically present: plasma glucose greater than 250 mg/dl (13.9 mmol/L), anion gap of greater than 10 mEq/L, the presence of ketonuria or ketonemia, serum bicarbonate below 18 mEq/L, and arterial pH less than 7.30. DKA is graded as mild, moderate, or severe, with increasing severity associated with lower pH and deterioration in mental status. Arterial blood gas measurement should be obtained at the outset; measurements of venous blood pH can be used to track the resolution of the acidosis thereafter.
| Diagnostic Criteria and Classification | DKA | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | HHS | |
| Plasma glucose (mg/dl) | > 250 | > 250 | > 250 | > 600 |
| Arterial pH | 7.25–7.30 | 7.00 to < 7.25 | < 7.00 | > 7.3 |
| Serum bicarbonate (mEq/l) | 15–18 | 10 to < 15 | < 10 | > 15 |
| Urine ketone | Positive | Positive | Positive | Small |
| Serum ketone* | Positive | Positive | Positive | Small |
| Effective serum osmolality (mOsm/l) | Variable | Variable | Variable | > 320 |
| Anion gap (mEq/l) | > 10 | > 12 | > 12 | < 12 |
| Mental status | Alert | Alert/drowsy | Stupor/Coma | Variable |
As 3-beta-hydroxybutyrate is the most abundant ketone in the early stages of DKA, but is not measured in the nitroprusside assay for ketones, assessment should also include direct measurement of 3-beta-hydroxybutyrate when available; a level over 3 mmol/liter is considered clinically significant. Although a glucose of at least 250 mg/dl is included among the diagnostic criteria, patients who are pregnant or who have a high glomerular filtration rate can be in DKA at glucose levels under this threshold.
Precipitating factors fall into two categories: lack of insulin or development of another illness. Approximately one-quarter of DKA admissions occur at the onset of diabetes. Although type 1 diabetes accounts for the majority of these initial presentations, as many as half of adult African Americans and Hispanics with new-onset diabetes presenting in DKA may actually have type 2 diabetes, and ultimately may be able to discontinue insulin therapy. In the patient with a prior history of diabetes, poor adherence to insulin is the most common precipitating factor, particularly in adolescence. Technical problems with pumps, improper storage of insulin, or psychological or cognitive disorders should also be considered.
Among intercurrent illnesses, infection is a particularly common precipitating factor and must be assiduously excluded. Other causes include myocardial infarction, stroke, pancreatitis, and medications such as glucocorticoids and antipsychotic medicines.
DKA is not the only cause of an anion gap acidosis. Differential diagnoses include alcoholic ketoacidosis, starvation ketosis, severe chronic renal failure, lactic acidosis, and toxic ingestions such as salicylate, methanol, ethylene glycol, and paraldehyde.
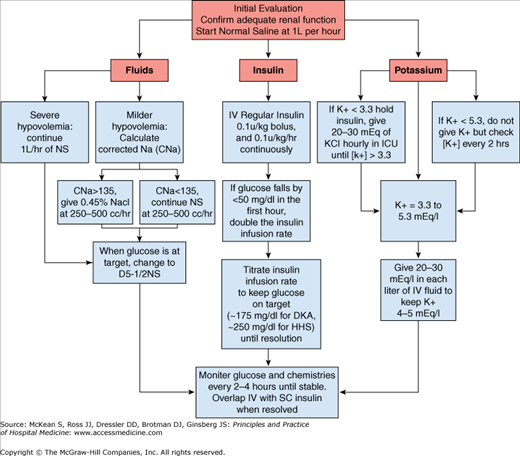
Treatment of diabetic ketoacidosis should simultaneously address each of the components that contribute to the syndrome: dehydration, hyperglycemia, electrolyte loss, and acidosis. A stepwise approach can facilitate management of these patients (Figure 148-1). Most patients with DKA are managed initially in critical care units, but local experience may facilitate management in a monitored general unit.
Patients with diabetic ketoacidosis are invariably dehydrated. Initial fluid resuscitation in the average adult without cardiac dysfunction should be 1 liter of 0.9% sodium chloride, infused over one hour. Subsequent fluid replacement will depend on the degree of dehydration and corrected sodium level. Equations that are helpful in guiding therapy are presented in Table 148-2.
| Calculation | Equation | Normal Range |
|---|---|---|
| Corrected Na (CNa) | Na (in mEq/L) + .016 (glucose [in mg/dl] – 100) | 135–145 mEq/L |
| Effective osmolarity | 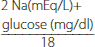 | 280–290 mOsm/L |
| Water deficit | 0.6 (wt in kg) ([CNa-140]/140) | < 0.5 L |
After the first liter of normal saline is infused, hypotonic fluids are appropriate if either corrected sodium level or serum osmolarity are elevated. Typical total body water deficit in DKA in adults is 6 liters, which should be replaced in the first 24 to 36 hours. Once serum glucose falls to between 200 and 250 mg/dl (11.1–13.9 mmol/L), dextrose should be added to the intravenous fluids to maintain glucose between 150 and 200 mg/dl (8.3–11.1 mmol/L).
Intravenous insulin therapy is the standard of care for the management of hyperglycemia in DKA, although subcutaneous insulin algorithms have been used successfully in some patients with mild and uncomplicated DKA. The goal of intravenous insulin therapy is to lower serum glucose by approximately 50 to 70 mg/dl (2.8–4.2 mmol/L) per hour. This is usually accomplished with a 0.1 U/kg intravenous bolus of regular insulin, followed by a 0.1 U /kg/hr insulin infusion, with the drip rate adjusted as needed to achieve the desired decrease in glucose. A bolus dose of insulin may not be necessary with an infusion rate of 0.14 U/kg/hr.
Intravenous insulin should be continued until ketoacidosis has resolved. The current American Diabetes Association consensus criteria suggest that the patient can be transitioned to subcutaneous insulin when the glucose is below 200 mg/dl (11.1 mmol/L), the serum bicarbonate is greater than 18 mEq/L, and the venous pH is greater than 7.30. In addition, intravenous insulin should not be stopped prior to normalization of the anion gap. Urinary ketosis may remain detectable after clearance of serum ketones, and therefore is an unreliable indicator for resolution of the acidosis.
Once DKA has resolved, intravenous insulin should be continued for two to four hours after the first subcutaneous dose of basal and rapid-acting insulin; premature discontinuation of the insulin infusion is the most common reason for recurrence of DKA in the hospitalized patient. Subcutaneous insulin must be dosed to ensure that both basal needs and prandial needs are met when the patient resumes eating. A well-established outpatient insulin dose can be used to determine the insulin replacement strategy in patients with known diabetes. Patients new to insulin can be started on a weight-based dose of 0.5–0.6 U/kg/day, divided into 0.25 U/kg/day of long-acting insulin for basal requirements and 0.1 U/kg of a rapid-acting insulin before meals.
Acidosis tends to raise serum potassium, even when total body potassium stores are depleted. Intravenous insulin, fluid replacement, and correction of acidosis lower serum potassium and can precipitate serious hypokalemia. In the absence of hyperkalemia, between 20 and 30 mEq of potassium should be added to each liter of intravenous fluids during treatment of DKA. Intravenous insulin treatment in a patient who is hypokalemic can be dangerous, so if it is less than 3.3 mEq/L at the initial evaluation, potassium should be administered through a central line and the patient should be monitored in the intensive care unit Adequate renal function should be established prior to starting potassium replacement.
Bicarbonate is not recommended for the management of acidosis in patients with DKA as long as the initial pH is greater than 7.0, as it may provoke hypokalemia in adults and cerebral edema in children. Judicious use of bicarbonate may be helpful for patients whose pH is less than 7.0.
Serum phosphate may be normal or high at the time of presentation of DKA, despite total body phosphate deficits. Serum phosphate falls rapidly with treatment. Aggressive phosphate replacement in DKA is generally not recommended, because of concerns of precipitating hypocalcemia and an absence of proven efficacy. If phosphate is below 1 mg/dL, it can be replaced in intravenous fluids as potassium or sodium phosphate at a dose of 20–30 meq infused over 6–12 hours. When the patient is eating and drinking again, milk is an excellent source of phosphate.
Cerebral edema is the most feared complication of DKA treatment. It is extremely rare in adults, but occurs in up to 1% of episodes in children and is associated with a high mortality in this group. A high rate of initial fluid resuscitation or bicarbonate therapy has been associated with cerebral edema, but its etiology has not been clearly determined. Treatment with intravenous mannitol is recommended if cerebral edema develops, but outcomes are often poor.
Patients should be educated about the dangers of insulin omission and guidelines for insulin adjustment during illness. In the hospitalized patient, diabetic ketoacidosis is preventable with adequate monitoring and should never occur de novo. DKA can develop in hospitalized patients with diabetes if glucose levels are not monitored, or when only correctional (sliding scale) insulin is prescribed, omitting basal insulin from the patient’s regimen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree