KEY POINTS
Aggressive intravenous resuscitation with fluids and blood, and airway protection are crucial in the management of the acutely bleeding patient.
Endoscopy should be performed with therapeutic intent for both upper and lower gastrointestinal bleeding.
Pharmacologic therapy should be used as an adjunct to endoscopic therapy.
An early team approach, involving medical, radiologic, and surgical personnel, should be implemented.
In the setting of severe bleeding or bleeding refractory to endoscopic therapy, angiographic and surgical therapies should be instituted promptly.
Gastrointestinal (GI) hemorrhage continues to be a frequent indication for intensive care management, with estimated rates of acute hospitalizations of 375,000 annually in the United States.1 Upper GI (UGI) bleeding has continued to predominate, with lower GI (LGI) bleeding constituting approximately 25% of all GI bleeding.2 Despite improved diagnostic and therapeutic modalities in the last two decades, the mortality rates for upper and lower GI hemorrhage have demonstrated different trends. Mortality from UGI bleeding has remained stable at 10%,3-6 which could be explained by an aging population with a significantly higher GI bleeding mortality due to comorbid conditions. In contrast, the mortality from LGI bleeding has decreased dramatically despite an aging population, and this is probably due to more aggressive diagnostic and therapeutic endoscopic intervention.
The management of GI hemorrhage in the ICU is multidisciplinary, involving the intensivist, gastroenterologist, radiologist, and surgeon. A successful outcome relies on effective fluid resuscitation, maintenance of adequate perfusion pressure, prompt hemostasis, monitoring of end-organ function, and prevention of multiple-organ failure.
CLINICAL CONSIDERATIONS
Multiple studies focusing primarily on nonvariceal UGI bleeding have been designed to define prognostic factors for GI bleeding and to identify high-risk patients.7-10 A common and pivotal feature of these studies is the combined use of clinical variables and endoscopic findings to guide risk stratification, thereby stressing the importance of integrating clinical and endoscopic information for optimal decision making. Table 105-1 outlines the clinical and endoscopic indicators associated with an increased risk of rebleeding and higher mortality. Other studies identified similar prognostic indicators for LGI bleeding.11-13
Adverse Clinical and Endoscopic Prognostic Indicators
| Clinical indicators |
| Age >60 years |
| Severe comorbidities |
| Onset of bleeding during hospitalization |
| Emergency surgery |
| Clinical shock |
| Red blood emesis or NG aspirate |
| Requiring >5U PRBCs |
| Endoscopic indicators |
| Major stigmata: active bleeding, visible vessel, adherent clot |
| Ulcer location: posterior duodenal bulb, higher lesser gastric curvature |
| Ulcer size >2 cm in diameter |
| High-risk lesions: varices, aortoenteric fistula, malignancy |
GI bleeding is divided into UGI and LGI bleeding based on its location proximal or distal to the ligament of Treitz at the junction of the duodenum and jejunum. UGI bleeding commonly presents with hematemesis and/or melena, and a nasogastric (NG) lavage that yields blood or coffee-ground material supports the diagnosis. However, it is important to note that a negative or bile-stained NG aspirate (indicating an open pylorus) does not exclude a UGI source because the bleeding may be intermittent.14,15 In comparison, hematochezia is usually the presenting sign of an LGI source. These distinctions based on presenting signs are not absolute because melena can be seen with proximal LGI bleeding and hematochezia can be present due to massive, brisk UGI bleeding.
Table 105-2 outlines the initial evaluation and management for GI hemorrhage.
Initial Management of Gastrointestinal Hemorrhage
| Maintain two large-bore IV catheters (14- or 16-gauge peripheral IV/central line) |
| Fluid resuscitate with crystalloids to maintain hemodynamic stability |
| Transfuse packed red cells to maintain hematocrit >30% |
| Urgent endoscopy with therapeutic intention for refractory hypotension/shock |
| Acid-suppression therapy with IV H2RAs or PPIs after endoscopic treatment |
| Platelet transfusion and fresh frozen plasma/recombinant factor VIIa to correct thrombocytopenia and coagulopathy |
| ECG in patients at risk for myocardial ischemia |
| A nasogastric tube should be inserted if the patient has hematemesis |
| CVP or Ppw monitoring may be helpful if variceal bleeding is suspected. A CVP <10 mm Hg may help prevent recurrent variceal bleeding |
| Splanchnic vasoconstrictors (octreotide/terlipressin) in variceal bleeding |
| Empirical antibiotics in variceal bleeding |
| Consultation with interventional radiology and surgery |
Hemodynamic: Regardless of the etiology and site of GI hemorrhage, the initial management should be directed at maintaining hemodynamic stability by restoring intravascular volume. Intravenous access with two large-bore IV catheters should be maintained at all times. In the presence of hypotension or hypovolemic shock, prompt fluid resuscitation with crystalloids and packed red blood cells is essential. Monitoring end-organ perfusion and preventing ischemic organ injury improve survival. In particular, coronary and renal perfusion should be assessed. An electrocardiogram should be obtained in patients at risk for myocardial ischemia, and renal laboratory parameters and urine output should be followed to assess for possible prerenal azotemia, acute renal failure, and (in cirrhotics) hepatorenal syndrome. In the subset of patients with suspected variceal hemorrhage, central venous pressure (CVP) monitoring may be useful to prevent sustained portal hypertension and recurrent bleeding following aggressive fluid replacement, with a goal to maintain a euvolemic status. When left-sided heart failure coexists, monitoring of pulmonary artery wedge pressure (Ppw) may facilitate aggressive fluid resuscitation while reducing the risk of cardiogenic pulmonary edema.
Gastrointestinal/Endoscopic: Prompt identification and hemostasis of the source of GI hemorrhage are essential in improving patient outcome. In the event that initial fluid resuscitation establishes hemodynamic stability, endoscopy may be performed under stable conditions within the first 24 hours of the bleed.16 However, more emergent endoscopy with therapeutic hemostatic intent should be considered for patients with UGI hemorrhage who cannot be stabilized hemodynamically with intravascular volume resuscitation and continue to bleed. For lower GI hemorrhage, some studies have suggested that early colonoscopy can identify the source of bleeding and improve outcome in patients with lower GI bleeding.17,18 However, other studies have not shown a difference in terms of clinical outcomes and cost between urgent colonoscopy as compared with routine elective colonoscopy in patients with serious lower GI bleeding.19 If there is massive lower gastrointestinal hemorrhage, a bleeding scan followed by angiography should be considered, as this allows identification of the source of bleeding and allows for therapeutic intervention, without the need for bowel prep that often limits the utility of colonoscopy. Absolute contraindications to endoscopy include suspected GI perforation, acute uncontrolled unstable angina, severe coagulopathy, untreated respiratory decompensation, and severe patient agitation. Apart from perforation, other conditions contraindicating endoscopy can be corrected, following which endoscopy should be performed. In the face of massive exsanguination, angiography or emergent surgical intervention (possibly facilitated by intraoperative endoscopy) should be considered instead of endoscopy.
Recent studies have suggested that the prokinetic agent erythromycin, given as a single intravenous dose of 250 mg prior to an EGD, improves visualization and diagnosis,20 and should be considered for patients expected to have substantial amounts of blood and clots in the stomach.16 However, prokinetics should not be used routinely, as they were not shown to affect important outcomes such as units of blood transfused, length of hospital stay, or the need for surgery.21
In the setting of nonvariceal UGI bleeding, acid suppression with proton-pump inhibitors is recommended prior to endoscopy, as this may downstage the endoscopic lesion and decrease the need for endoscopic intervention. An intravenous bolus followed by continuous-infusion PPI therapy should be used to decrease rebleeding and mortality in patients with high-risk stigmata who have undergone successful endoscopic therapy.16,22 In the setting of variceal bleeding, pharmacologic therapy with a splanchnic vasoconstrictor such as octreotide and empiric antibiotic therapy should be initiated.
Hematologic: In order to ensure adequate oxygen-carrying capacity in the circulation and to prevent end-organ ischemia, the hematocrit should be maintained above 30%. It should be noted that the initial hematocrit after an acute GI bleed can be misleading because acute hemorrhage produces loss of whole blood, and the hematocrit does not change initially because the initial loss of plasma and erythrocytes is equivalent. Within 24 to 72 hours of the initial bleed, plasma is redistributed from the extravascular to the intravascular space, thus resulting in a dilution of the red cell mass and a fall in the measured hematocrit. Intravenous hydration with crystalloids compounds this dilutional anemia so that red cells should be replaced promptly. In most instances, there is sufficient time to allow typing and cross-matching of red cells; however, in the setting of massive exsanguination, the transfusion of non–cross-matched type-specific blood may be necessary. In the presence of thrombocytopenia, platelets must be transfused to maintain the count above 60,000/µL. Any existing coagulopathy should be corrected with fresh frozen plasma; however, this should not delay endoscopy in most cases. Studies have demonstrated the ability of recombinant activated factor VIIa (rFVIIa) to rapidly correct severe coagulopathy in hepatic failure23,24; however, two large studies have not shown a benefit for factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis.25,26 During the process of aggressive intravascular volume resuscitation, fluids and blood products should be warmed to prevent the development of a cold coagulopathy, and the core body temperature should be maintained above 35°C.
Pulmonary: In the presence of active hematemesis, a nasogastric tube should be placed to decrease the risk of aspiration. Endotracheal intubation should be performed for airway protection and to decrease the risk of aspiration in the following situations: (1) in the presence of active hematemesis and decreased mental status, (2) prior to an emergent EGD for active hematemesis, and (3) prior to insertion of an esophageal tamponade tube. In the setting of shock, intubation and full mechanical ventilatory support are indicated to decrease the oxygen consumption of the respiratory apparatus. During the performance of an EGD, significant hypoxemia may occur, especially in elderly patients and in those with moderate to severe obstructive pulmonary disease (defined as an FEV1/FVC ratio of less than 0.6).27 Probable causes include hypoventilation due to sedative agents, the partial airway obstruction produced by the endoscope, and aspiration of gastric contents, resulting in bronchospasm and ventilation-perfusion mismatch.
Consultation: Radiology and surgery should be consulted early in the course of management. In the setting of nondiagnostic upper or lower endoscopy, radiologic modalities offer diagnostic and therapeutic options to achieve hemostasis. Emergent surgical intervention is indicated in the exsanguinating patient who may not be stable enough for endoscopic or radiologic evaluation.
When endoscopic evaluation is unable to identify the cause of bleeding, the two diagnostic radiologic modalities available are angiography and radionuclide scans. As outlined later, angiography with embolotherapy or selective infusion of vasoconstrictors offers therapeutic options as well.
The angiographic diagnosis of acute arterial hemorrhage is based on visualization of extravasated contrast material in the gastrointestinal lumen. Therefore, it only identifies the bleeding site if active bleeding is occurring when the study is performed. In addition, the rate of bleeding must be brisk, in the range of 0.5 to 1 mL/min. In the case of brisk UGI bleeding, angiography may demonstrate a bleeding site in 75% of patients, with most bleeding episodes originating from a branch of the left gastric artery. In the setting of LGI bleeding, the average diagnostic yield is decreased to about 60%, with diverticular disease and vascular ectasia being the most common findings.
Radionuclide studies occasionally aid in the detection of the bleeding site. The current radionuclide scan of choice is the 99 mTc-pertechnetate-labeled red blood cell scan. The radionuclide scan offers the ability to detect rates of bleeding of less than 0.5 mL/min, and the 48-hour stability of the tagged red blood cells allows repeated nuclear imaging for 1 to 2 days following administration of the radionuclide in the setting of intermittent bleeding. However, a positive radionuclide study localizes the bleeding only to an area of the abdomen and cannot define the mucosal location of the bleeding site precisely. Therefore, a positive result should prompt a repeat endoscopy or angiography to localize the bleeding site precisely.
In most instances, the presentation of rebleeding is similar to that of the initial episode, and the source is identical to the site of the initial bleed. After hemostasis of the initial bleed following spontaneous cessation or therapeutic intervention, the patient should be monitored closely for rebleeding, especially in the presence of clinical and endoscopic indicators associated with an increased risk of rebleeding (see Table 105-1). Most patients who have undergone upper endoscopic hemostasis for high-risk stigmata should be hospitalized for at least 72 hours thereafter. Apart from the obvious signs of gastrointestinal blood loss characterized by melena, hematemesis, or hematochezia, more subtle signs of rebleeding may include tachycardia and hypotension owing to a decreasing intravascular volume. Therefore, continuous hemodynamic monitoring should be performed following initial hemostasis, and invasive monitoring with a central venous catheter or an arterial line may be considered for the patient at high risk for rebleeding. A falling serum hemoglobin concentration on serial measurements may suggest a recurrent bleed. The management of rebleeding should include immediate repeat endoscopic intervention targeted at the initial lesion, followed by radiologic or surgical intervention, if necessary. Specific pharmacologic and endoscopic interventions for long-term secondary prophylaxis against rebleeding will be discussed in the sections that follow.
UPPER GASTROINTESTINAL HEMORRHAGE
VARICEAL HEMORRHAGE
Variceal hemorrhage presents as a symptom of decompensated cirrhosis in as many as 50% of patients and accounts for about one-third of all deaths related to cirrhosis. Mortality is related to hepatic disease severity, as defined by the Child-Pugh classification (Table 105-3), with an overall mortality estimated at 50%. There are two distinct phases in the course of variceal hemorrhage. In the first phase, defined by the initial episode of active hemorrhage, only 50% of patients stop bleeding spontaneously (in contrast to nonvariceal hemorrhage, in which 90% cease spontaneously). The initial bleed is followed by a second phase of an approximately 6-week duration, defined by a high risk of recurrent hemorrhage, with the greatest risk of rebleeding being within the first 48 to 72 hours.
Child-Pugh Classification of Hepatic Disease Severity
| Points Assigned | |||
|---|---|---|---|
| Parameter | 1 | 2 | 3 |
| Ascites | Absent | Slight | Moderate |
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| INR | <1.7 | 1.8-2.3 | >2.3 |
| Encephalopathy | None | Grade 1-2 | Grade 3-4 |
| Total Score (Five Parameters) | Child-Pugh Stage | ||
| 5-6 | A | ||
| 7-9 | B | ||
| 10-15 | C | ||
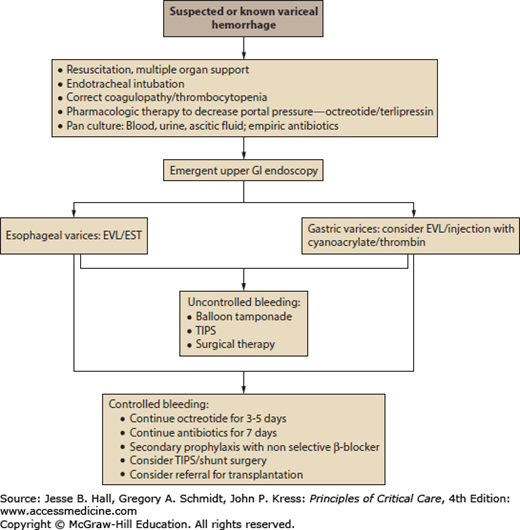
The management of variceal bleeding is outlined in Figure 105-1. In addition to multiorgan ischemic injury from hypoperfusion, variceal hemorrhage in the setting of cirrhosis predisposes the patient to specific derangements, including hepatic encephalopathy, type 1 hepatorenal syndrome, and systemic infection. These processes contribute to the high mortality associated with variceal bleeding, and therefore, the management should address these issues in addition to achieving hemostasis and hemodynamic stability.
Cardiopulmonary: Fluid resuscitation should be aimed at achieving a euvolemic status because this approach prevents persistent portal hypertension and recurrent variceal bleeding.28 To this end, invasive hemodynamic monitoring with a central venous catheter can be used to guide fluid therapy. In the setting of hypotension that is refractory to fluid resuscitation, a peripheral vasoconstrictor such as norepinephrine should be the vasoactive agent of choice. Agents that have β2-agonist activity, such as dopamine, should be avoided because they potentially could cause splanchnic vasodilation and therefore worsen the variceal bleed. Splanchnic vasoconstrictors such as octreotide and terlipressin (discussed later) can have a beneficial effect on systemic blood pressure by diverting blood away from the splanchnic circulation. Endotracheal intubation for airway protection is critical, especially in the setting of encephalopathy, active hematemesis, or emergent endoscopy.
Infection: Cirrhosis is characterized by cellular and humoral immune dysfunction, and increased bacterial translocation from the gut into the bloodstream, facilitating the development of infections. The most common bacterial infections are caused by gram-negative bacteria, producing spontaneous bacterial peritonitis (25%), urinary tract infections (20%), pneumonia (15%), and bacteremia (12%).29,30 The presence of infection has been associated with failure to control the initial bleed and an increase in the recurrence of rebleeding, likely owing to the induction of a hyperdynamic circulation and increased portal pressure.31,32 A recent meta-analysis and systematic review of studies regarding the use of prophylactic antibiotics in cirrhotics with upper gastrointestinal bleeding concluded that antibiotics reduced bacterial infections, all-cause mortality, bacterial infection–related mortality, rebleeding events, and hospitalization length29; therefore, the administration of antibiotics in the setting of variceal bleeding has become the standard of care. Although most of the pertinent studies include a quinolone, the optimal choice and duration of antibiotic therapy have not been defined, and therefore, the choice of empiric antibiotic therapy should be institution specific. One study from Spain showed that intravenous ceftriaxone is more effective than oral norfloxacin33; however, this was likely secondary to high incidence of quinolone resistance in that patient population. The choice of nonfluoroquinolone antibiotic therapy is an important consideration in cirrhotic patients who have been previously on fluoroquinolones for the prevention of spontaneous bacterial peritonitis (SBP). It is recommended that intravenous antibiotics be started initially, followed by a switch to an oral formulation once the bleeding has been stabilized, for a total duration of 7 days. Prior to initiating antibiotic therapy, blood, urine, and (if indicated) ascitic fluid cultures should be obtained.
Hematologic: In acute variceal bleeding, both under- and overtransfusion should be avoided.34 Undertransfusion can lead to tissue hypoxia, and overtransfusion can lead to rebound portal hypertension, and possibly worsening of the acute bleeding episode. One randomized prospective study showed that a restrictive strategy of blood transfusion with a hemoglobin threshold of 7 g/dL resulted in decreased transfusion requirements, with a similar incidence of side effects and survival as compared to a liberal strategy of blood transfusion with a hemoglobin threshold of 9 g/dL.34Failure to control bleeding was higher in the liberal strategy group. Another recent randomized prospective study showed that a restrictive transfusion strategy significantly improved outcomes in patients with acute upper gastrointestinal bleeding, and especially in the subgroup of patients with Child-Pugh cirrhosis class A and B.35 In general, a hemoglobin of 7 to 8 is safe except in patients with cardiac ischemia.36
In addition, fresh frozen plasma should be given to correct coagulopathy; however, this should not delay endoscopy. rFVIIa is used as a procoagulant that can rapidly correct severe coagulopathy associated with decompensated liver disease. Initial studies suggested that it can promote hemostasis in variceal hemorrhage.23,24 However, two large subsequent trials of rFVIIa in cirrhotic patients with upper gastrointestinal bleeding showed no benefit in controlling variceal hemorrhage, rebleeding rates or mortality,25,26 and therefore, this drug is currently not recommended in the setting of variceal bleeding.
Neurologic: In the setting of decompensated liver disease, variceal bleeding could induce or exacerbate hepatic encephalopathy (HE). Therefore, in the presence of decreased mental status during variceal bleeding, HE should be considered a potential etiology in addition to cerebral hypoperfusion, and empirical lactulose therapy via an NG tube or as an enema should be considered.
Hemostatic Therapy: Specific therapies aimed at arresting active bleeding include pharmacotherapy and endoscopic therapy. Multiple studies have proven the increased hemostatic efficacy of combined pharmacotherapy and endoscopic therapy over either treatment alone.
In the setting of variceal bleeding, pharmacologic agents are aimed at causing splanchnic vasoconstriction and reducing portal hypertension. Empiric pharmacotherapy should be initiated in suspected variceal hemorrhage prior to endoscopic diagnosis and intervention. Selective splanchnic vasoconstriction has the added advantage of diverting blood flow from the splanchnic circulation to the systemic circulation, thereby improving systemic blood pressure. In particular, improved renal perfusion could prevent or ameliorate the hepatorenal syndrome.
The current agent of choice in the United States is the somatostatin analog octreotide. Somatostatin and its analogs inhibit the release of vasodilator hormones such as glucagon, thereby indirectly causing splanchnic vasoconstriction and decreased portal inflow. Although octreotide has a longer half-life than somatostatin, its therapeutic efficacy is obtained only with a continuous infusion; the recommended dose is a 50 µg IV bolus, followed by an infusion of 50 µg/hour for 5 days. A meta-analysis of trials of octreotide has demonstrated improved control of bleeding compared with other therapies, including the previous agent of choice, vasopressin.37 Furthermore, the adverse extrasplanchnic vasoconstrictive effects observed with vasopressin, such as myocardial and cerebral ischemia, are not observed with octreotide.
Terlipressin is a long-acting vasopressin analogue that has received a favorable recommendation based on European studies, which report fewer side effects than vasopressin and an efficacy similar to that of octreotide and endoscopic sclerotherapy.38 In addition, a systematic review comparing trials of terlipressin with other pharmacotherapies identified terlipressin as the only pharmacologic agent that reduced mortality.39 More recently, the efficacy of terlipressin was shown to be similar to octreotide as an adjuvant therapy for the control of esophageal variceal bleed and in-hospital survival.40 Terlipressin alone is inferior to terlipressin combined with band ligation in the treatment of acute variceal bleeding without active bleeding at endoscopy.41 In conclusion, endoscopic band ligation combined with a somatostatin analogue (octreotide or terlipressin) remains the standard of care for acute variceal bleeding.
Following the administration of pharmacotherapy, emergent endoscopy with therapeutic hemostatic intent is imperative. As outlined in Figure 105-1, endoscopic evaluation can localize the source of the variceal bleed to an esophageal or gastric varix. This is an important distinction because, while esophageal variceal bleeding is amenable to endoscopic therapy, gastric variceal bleeding may require more aggressive salvage measures, as outlined below.
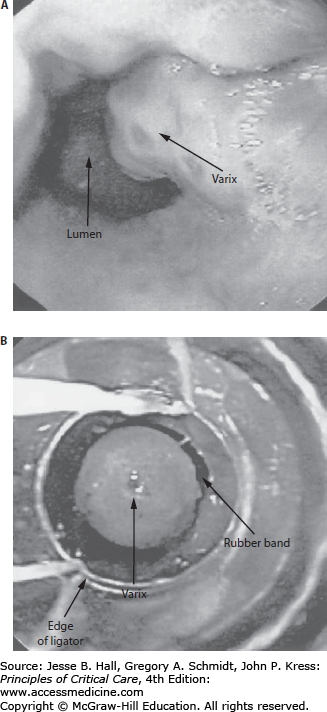
Endoscopic therapy is based on the interruption of blood flow through the venous collateral system lining the distal esophagus and gastric cardia using either stimulation of thrombosis (eg, sclerotherapy) or immediate occlusion (eg, band ligation). The two established forms of endoscopic therapy are endoscopic sclerotherapy (EST) and endoscopic variceal band ligation (EVL). While sclerotherapy involves the intravariceal or paravariceal injection of a sclerosant (eg, sodium morrhuate), band ligation involves the placement of small bands around varices in the distal 5 cm of the esophagus (Fig. 105-2). A meta-analysis has shown that EVL is superior to EST in initial hemostasis, obliteration of varices, rates of recurrent bleeding, complications, and mortality.42 EVL was also shown to be superior to sclerotherapy when both treatments are combined with a somatostatin analogue,43 and EVL is now the endoscopic treatment of choice for acute variceal hemorrhage. However, a technical challenge during use of the band ligator apparatus is the decreased endoscopic field of view in a setting already complicated by active hemorrhage. Therefore, EST may be indicated in the setting of poor initial visualization, followed later by definitive EVL treatment.
Gastric Varices: While the preceding endoscopic interventions are effective in esophageal variceal bleeding, gastric variceal bleeding presents a technical challenge. Gastric varices are located deeper in the submucosa, where EVL and EST are not successful in obtaining sustained hemostasis. Initial studies reporting successful hemostasis with intravariceal injection of cyanoacrylate tissue glue44,45 and thrombin46 suggest novel approaches to endoscopic intervention in gastric variceal bleeding. However, these therapies need further validation, are only done in specialized centers, are not FDA approved, and can lead to serious complications such as embolism, infection, and death. In the setting of gastric variceal bleeding, EVL may be attempted to obtain initial hemostasis. However, given the limited success of endoscopic hemostasis, the emergent application of nonendoscopic interventions should be anticipated, which may include balloon tamponade (using the Linton-Nachlas tube), transjugular intrahepatic portosystemic shunt (TIPS), and surgery.
Complications of Endoscopy: Most complications have been associated with EST, and the advent of variceal band ligation (EVL) has decreased the incidence of complications significantly after therapeutic endoscopy. Following endoscopic intervention, local complications include ulceration, dysmotility, and stricture formation, and regional complications include esophageal perforation and mediastinitis. In addition, both EST and EVL increase the risk of developing portal hypertensive gastropathy (PHG) and its bleeding sequelae because blood is shunted away from the venous system of the gastroesophageal junction to that of the gastric mucosa. Bleeding from PHG is characterized by a diffuse, slow bleed from the gastric mucosa that typically is not amenable to localized endoscopic therapy.
In the event that hemostasis cannot be achieved by initial endoscopic therapy or there is evidence of rebleeding after initial hemostasis, a repeat trial of endoscopic therapy may be attempted. However, following a second failed endoscopic trial, nonendoscopic interventions need to be implemented emergently. These interventions may include balloon tamponade, TIPS, and surgical therapy.
Balloon Tamponade: Tamponade tubes (such as the Sengstaken-Blakemore or Minnesota tubes) effectively achieve short-term hemostasis by compressing the gastric and distal esophageal mucosa. In most cases, tamponade is effective after inflating only the gastric balloon. Endotracheal intubation is imperative prior to insertion of tamponade tubes to decrease the risk of aspiration. Once the airway is secured, the tube can be passed either nasally or orally to the stomach. The gastric balloon should be inflated only partially (with approximately 30 mL of air) pending radiographic confirmation of correct placement because inadvertent inflation of the balloon in the esophagus can lead to esophageal perforation. Once adequate positioning is ensured, the gastric balloon can be inflated fully according to the manufacturer’s recommendations (gastric balloons are inflated to predetermined volumes, whereas the esophageal balloon component is inflated according to pressure). If hemostasis is not achieved by isolated inflation of the gastric balloon, the esophageal balloon can be inflated to 35 mm Hg, a pressure exceeding the intravariceal pressure. Following inflation, traction should be applied to the apparatus at the insertion site to maintain proper positioning. A maximum duration of 48 hours is recommended for variceal compression because prolonged tamponade can lead to esophageal wall ischemia. A nasogastric tube inserted above the esophageal balloon is mandatory to prevent aspiration of oropharyngeal secretions that collect above the inflated apparatus (eg, Sengstaken-Blakemore tube), unless the tube has its own lumen for esophageal suction (eg, Minnesota tube). A major limitation of tamponade therapy is the high risk of rebleeding following deflation of the balloon. Furthermore, given the serious complications of pulmonary aspiration and esophageal ulceration and perforation, this mode of therapy should be performed by skilled personnel and generally as a temporizing step while planning definitive treatment such as a TIPS. For gastric varices, balloon tamponade should be attempted using the Linton-Nachlas tube which has a 600-mL volume single gastric balloon that seems to be more effective in controlling fundal variceal bleeding.47
Transjugular Intrahepatic Portosystemic Shunt: Following temporary stabilization with balloon tamponade, further definitive treatment to achieve hemostasis involves the creation of an artificial vascular shunt between the systemic and portal circulation in order to decompress the variceal vasculature. This can be accomplished surgically (discussed below) or via TIPS, which offers a less invasive method for obtaining a vascular shunt. The TIPS consists of an expandable metallic stent placed intrahepatically between portal and hepatic veins using radiologically guided access. Traditionally, TIPS has been recommended as secondary prophylactic therapy for variceal bleeding in the setting of mild to moderate liver disease, and advanced cirrhosis has been regarded as a contraindication to TIPS therapy due to increased mortality after TIPS in this setting. However, recent studies with favorable hemostasis and mortality data have supported the use of emergent TIPS as salvage therapy for refractory variceal hemorrhage, even in advanced cirrhosis.48,49 Therefore, when variceal hemorrhage is refractory to pharmacologic and endoscopic therapy, urgent TIPS therapy should be considered irrespective of the severity of hepatic disease. The hemodynamic benefits of TIPS therapy can be attributed not only to portal decompression and variceal hemostasis, but also to increased venous return due to intravascular mobilization of any existing ascites. Additional beneficial effects of TIPS therapy include treatment of refractory ascites and the hepatorenal syndrome.
Recently, early TIPS has been investigated to prevent variceal rebleeding and improve outcome early after EBL therapy in patients with Child-Pugh class C or those in class B who have persistent bleeding at endoscopy.50 Early TIPS (within 72 hours of admission) was compared with standard therapy (continuation of vasoactive drug therapy), followed after 3 to 5 days by treatment with propranolol or nadolol and long-term EBL. Patients in the standard therapy group received TIPS if needed as rescue therapy. The early TIPS group was more likely to remain free of rebleeding events compared to the standard therapy group (97% vs 50%; p < 0.001). The 1-year survival was higher in the early TIPS group compared to the standard therapy group (86% vs 61%; p < 0.001).50 In light of the findings in this study, early TIPS could be considered in patients with advanced cirrhosis at high risk of variceal rebleeding; however, larger multicenter trials are needed to validate this study’s findings.
Surgical Therapy: Since the advent of TIPS as salvage therapy for refractory variceal hemorrhage, there has been a reduction in the need for surgical intervention. However, shunt surgery for portal decompression is indicated for variceal hemostasis in patients with preserved hepatic synthetic function (ie, Child-Pugh A disease).51 Esophageal transection with or without devascularization may be another surgical option in massive exsanguination refractory to other interventions.
Shunt operations can be divided into (1) nonselective shunts (eg, portocaval shunts) that decompress the entire portal system and divert all blood flow away from the portal vein and (2) selective shunts (eg, distal splenorenal shunt) that compartmentalize the portal tree into a decompressed variceal system and a hypertensive superior mesenteric vein that maintains sinusoidal perfusion. A selective shunt is the preferred operation because portocaval shunts significantly alter vascular anatomy and therefore complicate future liver transplant surgery. In addition, emergency portocaval shunts are associated with a higher rate of thrombosis and shunt failure.
Distal splenorenal shunt was found to be similarly efficacious in the control of refractory variceal bleeding in Child-Pugh class A and B patients compared to TIPS.52 The reintervention rate was higher in the TIPS group, mainly due to TIPS occlusion. However, the TIPS used in this study were the older uncoated stents, which are known to occlude more frequently than the currently used coated stents.
Distal esophageal transection in the setting of massive variceal exsanguination may control bleeding, but mortality remains above 80%. Since the transection does not address the underlying portal hypertension, varices recur after a variable period, and rebleeding should be anticipated. Esophageal transection with devascularization of the gastroesophageal junction (Sugiura procedure) may be considered in patients who have an absolute contraindication to shunt surgery, such as extensive thrombosis in the portal venous circulation involving the splenic, superior mesenteric, and portal veins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree