An assessment of active bleeding (and gastric decompression) can be determined by nasogastric (
NG) tube placement, although the role of tube placement is controversial. A meta-analysis of adult patients with
GI bleeding revealed that blood or coffee ground material in an
NG aspirate has a 44% sensitivity, 95% specificity, and positive likelihood ratio of 9.4 for
UGI bleeding (
7). In a study of 520 patients that underwent
NG aspirate prior to endoscopy for
UGI bleed, 15% had a clear aspirate but were found to have an upper
GI lesion on endoscopy (
8). Hence, a clear
NG aspirate does not exclude a

bleeding source proximal to the ligament of Treitz. Conversely, the presence of blood in the
NG aspirate generally confirms the diagnosis of
UGI bleeding in an otherwise consistent clinical context and provided swallowed blood can be ruled out. On balance, we favor the placement of an
NG tube in children in the
PICU with suspected
UGI bleeding to confirm the diagnosis and improve the conditions for endoscopy. Potential
complications of
NG tube insertion should be considered in patients with basil skull fracture, severe facial trauma, clotting disorders, esophageal varices, esophageal tumors, or esophageal surgery.
 Patients in a critical care unit are at high risk of gastrointestinal bleeding from stress-related mucosal ulcers and may benefit from prophylactic antisecretory therapy.
Patients in a critical care unit are at high risk of gastrointestinal bleeding from stress-related mucosal ulcers and may benefit from prophylactic antisecretory therapy. Initiate broad-spectrum antibiotic coverage and medical reduction of portal hypertension early in the course of acute variceal bleeding.
Initiate broad-spectrum antibiotic coverage and medical reduction of portal hypertension early in the course of acute variceal bleeding. bleeding source proximal to the ligament of Treitz. Conversely, the presence of blood in the NG aspirate generally confirms the diagnosis of UGI bleeding in an otherwise consistent clinical context and provided swallowed blood can be ruled out. On balance, we favor the placement of an NG tube in children in the PICU with suspected UGI bleeding to confirm the diagnosis and improve the conditions for endoscopy. Potential
bleeding source proximal to the ligament of Treitz. Conversely, the presence of blood in the NG aspirate generally confirms the diagnosis of UGI bleeding in an otherwise consistent clinical context and provided swallowed blood can be ruled out. On balance, we favor the placement of an NG tube in children in the PICU with suspected UGI bleeding to confirm the diagnosis and improve the conditions for endoscopy. Potential 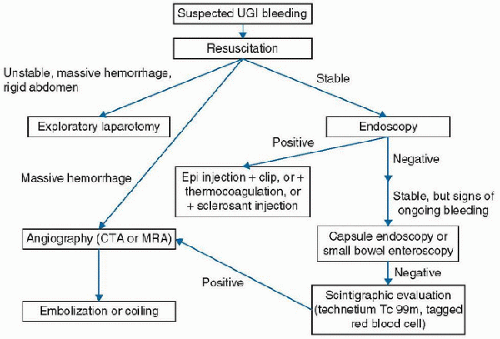
 difficile [C. diff] PCR). Fecal and gastric contents can be tested for the presence of hemoglobin using a guaiac-based test to detect occult bleeding. False-positive guaiac reactions occur with intake of red meat, raw fruits or vegetables, and other foods with peroxidase activity. Inflammatory markers (ESR and CRP) are useful when inflammatory bowel disease is considered.
difficile [C. diff] PCR). Fecal and gastric contents can be tested for the presence of hemoglobin using a guaiac-based test to detect occult bleeding. False-positive guaiac reactions occur with intake of red meat, raw fruits or vegetables, and other foods with peroxidase activity. Inflammatory markers (ESR and CRP) are useful when inflammatory bowel disease is considered.





