Figure 17.1
Laparoscopic pyloroplasty with longitudinal incision across pylorus (A duodenum, B pylorotomy, C stomach)
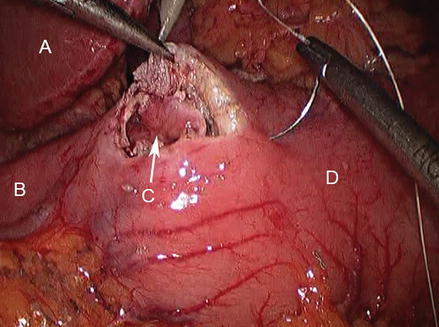
Figure 17.2
Closure of enterotomy proceeds transversely; retraction with atraumatic grasper replaces preplaced stay sutures (A liver, B duodenum, C pylorotomy, D stomach)
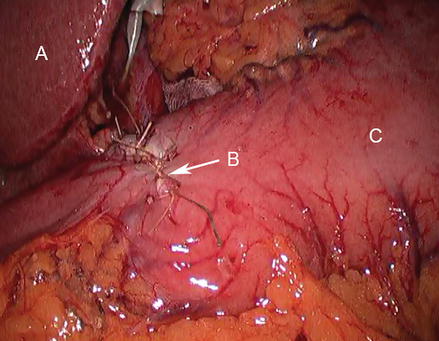
Figure 17.3
Completed pyloroplasty (A liver, B closed enterostomy, C stomach)
As with other laparoscopic foregut procedures, patient positioning is critical. Low lithotomy position allows the surgeon to position themselves with better access to the area of interest. The laparoscope monitor should be positioned at the head of the surgical table. The technique is similar to that described in the open case however may benefit from placement of a liver retractor to assist in visualization. An endostitch or similar endoscopic suturing device maybe used to assist in intracorporeal suturing of the enterotomy.
Regardless of the approach used, care should be taken to avoid narrowing the gastric outlet with the closure of the enterotomy. If the cause of gastric outlet obstruction is unclear luminal biopsy should be taken at the time of the procedure.
Finney Pyloroplasty
A Finney Pyloroplasty is better suited to more extensive pyloric narrowing and is conceptually similar to a side-to-side bowel anastomosis. This technique can be used either with an open or laparoscopic approach. Mobilization of the duodenum by a Kocher maneuver is essential to allow approximation of the suture line without undo tension. An incision is made along the anterior wall of the stomach starting 5 cm proximal to the pylorus and extending longitudinally 5 cm distal on the duodenum. A stay suture is placed at the pylorus superior to the incision; this will form the apex of the anastomosis. Another stitch then joins the distal end of the incision in the duodenum to the proximal end in the stomach thus approximating a cut edge of duodenum to a cut edge of stomach both posteriorly and anteriorly. These edges can then be anastomosed in one or two layers. Again care should be taken with two layer closure not to narrow the new lumen created.
Jaboulay Gastroduodenostomy
A Jaboulay gastroduodenostomy bypasses the area of pyloric narrowing and is best suited to cases where there is active inflammation at the pylorus or the proximal duodenum or an extensive segment of narrowing. Mobilization of the duodenum by a Kocher maneuver is also essential to allow approximation of the suture line without undo tension. Following mobilization of the duodenum the pylorus is left intact and the most proximal area of healthy appearing duodenum is approximated to the distal antrum with a back wall of sero-muscular interrupted silk sutures which will form the second layer of the posterior aspect of the anastomosis. The approximated gastric and duodenal walls are then incised and a full thickness anastomosis is performed using absorbable monofilament suture. The second layer of the anterior aspect of the anastomosis is then completed with interrupted silk Lembert stitches.
Graham Patch
Indications
Perforated peptic duodenal ulcers remain a common cause of pneumoperitoneum, necessitating emergent surgical treatment. Given the emergent nature of the patient’s presentation, as well as improvements in medical management of peptic duodenal ulcer disease, a Graham patch repair without any other surgical intervention to reduce gastric acid production is usually preferred to treat perforated duodenal ulcers. For patients with a perforated gastric ulcer, a partial gastrectomy is the optimal treatment, however if the patients clinical status will not allow a formal resection, a wedge resection may be performed. Given the risk that a gastric perforation represents a gastric cancer, simple closure is only an option when the patient’s condition is so poor that the surgeon judges it unsafe to proceed with either of the first two options.
A laparoscopic approach is safe when patient condition is stable and may decrease peri-operative morbidity [3]. Data from the National Surgical Quality Improvement Program demonstrated reduction in duration of inpatient stay with the laparoscopic approach; however that finding is tempered by the fact that patients offered the open approach may have worse general medical condition at baseline [4].
Surgical Technique – Graham Patch
The traditional approach to the procedure is through an open upper midline incision although this may now be approached with laparoscopy. The wall of the stomach and duodenum are inspected for evidence of perforation. Once the defect is visualized on the duodenum (Fig. 17.4), a healthy portion of omentum is identified and mobilized so it can reach the defect without tension. Interrupted full thickness stay sutures of silk, vicril or PDS are placed bridging the defect, taking care not to incorporate the posterior bowel wall (Fig. 17.5). These sutures are only approximated after the mobilized portion of omentum is brought on top of the perforation and then secured within the loop of the sutures as they are tied. The basic concept of the Graham Patch is to ‘patch’ the perforation with the omentum and not to close the defect by approximating it with the sutures. Attempts to close the perforation primarily may lead to duodenal stenosis or tearing of the inflamed tissues. Care is taken to avoid securing the omentum too tightly and compromising blood flow to the omental patch (Fig. 17.6). The abdominal cavity is then irrigated and suctioned clean.
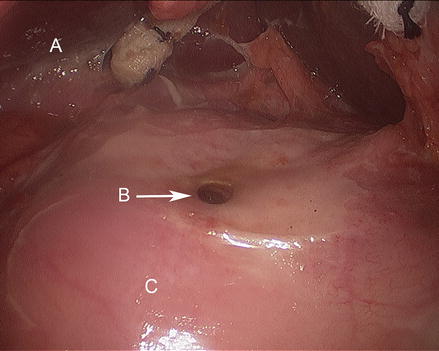
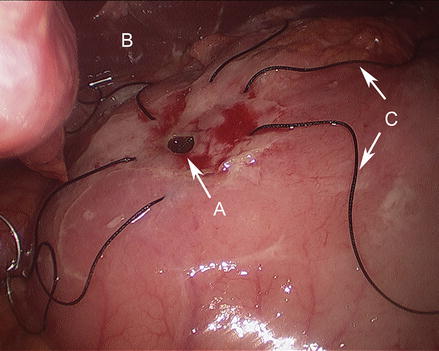


Figure 17.4
Duodenal ulcer with perforation (A liver, B duodenal perforation, C duodenum)

Figure 17.5
Stay sutures placed across ulcer defect with wide full thickness bites (A duodenal ulcer, B liver, C stay sutures)

Figure 17.6
Completed graham patch (A tongue of omentum)
As is seen in other cases of foregut surgery, positioning of the operator to reach the superior portion of the abdomen is essential. The patient positioning should be performed as described in the preceding section. Standard laparoscopic sutures with a SH needle and 2 or 3-0 sutures should be used; the endostitch or similar laparoscopic suturing device should be avoided as their needles are bulkier and may disrupted the inflamed tissues.
Vagotomy and Antrectomy
Indications
Typically reserved for recalcitrant peptic ulcer disease, the use of vagotomy and antrectomy is rare nowadays as medical therapy has improved. While vagotomy with antrectomy is quite effective in cases of persistent peptic ulcer disease with an estimated rate of recurrence of 0–2 %, it has potential for substantial morbidity. Surgery is reserved for those patients with recalcitrant disease or contraindication to medical therapy [5, 6]. When considering patients for surgical treatment of peptic ulcer disease, other causes of ulceration including neoplasm, Crohn’s disease and gastrinoma, should be excluded.
Vagotomy interrupts neural stimulation of acid secretion from parietal cells as promoted by acetylcholine release at the cell membrane. Three types of vagotomy were commonly used; truncal, selective or highly selective vagotomy. The pylorus is denervated with a truncal vagotomy, necessitating a gastric emptying procedure such as a pyloroplasty. Selective or highly selective vagotomies are almost never used in current surgical practice.
Antrectomy focuses on removing the site of gastrin production from antral G cells which subsequently stimulates acid secretion. The sites of mucosal injury and ulceration may also be resected with antrectomy, further promoting cure.
Surgical Technique – Vagotomy
Truncal Vagotomy
Truncal Vagotomy focuses on identifying and dividing the left and right vagus above the level of the crura. This procedure has moved to the forefront of surgical management of peptic ulcer disease as the incidence of elective surgery for this problem fades and with it surgeon experience with selective vagotomy. Of note, this procedure warrants a concurrent gastric emptying procedure as the pylorus is denervated along with the acid secreting parietal cells.
Via an upper midline laparotomy incision or laparoscopic surgery, the gastrohepatic ligament in incised and the esophageal hiatus is identified. Phrenoesophageal attachments are divided and the esophagus is encircled. Gentle spreading along the anterior esophagus will reveal the left vagus nerve. The right vagus is identified tracking posteriorly alongside the esophageal wall. Each nerve should be isolated, clipped proximally and distally and a 1 cm segment resected between the clips and sent to pathology for review. The nerves must be transected above the level of the hiatus so as to ensure the criminal nerve of grassi does not remain intact and cause recurrence.
Surgical Technique – Antrectomy
Antrectomy targets the site of gastrin production. At the completion of the procedure, the resected specimen should represent about 35 % of the total stomach. After visualizing the upper abdomen through an upper midline incision or via laparoscopy and retracting the liver, the gastrohepatic ligament is incised and the left gastric artery is ligated. The gastrocolic ligament is carefully dissected proximal to the main gastroepiploic arcade. Branching vessels to the greater curvature of the stomach are ligated. The dissection along the greater curve continues proximally to a point across the organo-axis from the incisura and distally to the pylorus. The stomach can then be transected proximally from the end of the proximal dissection along the greater curvature to the midpoint of the lesser curve. The antrum extends more proximally along lesser curve necessitating the more proximal resection on this aspect of the stomach. The antrum is then lifted anteriorly and dissected away from the pancreas posteriorly. The antrectomy is completed with transection of the duodenum just distal to the pylorus. A distal margin should be sent to confirm the presence of Brunner’s glands to avoid retained antrum. Kocherization of the duodenum may be a reasonable step prior to duodenal transection depending on the plan for reconstruction of continuity. Gastrointestinal continuity is restored either with a gastroduodenostomy (Billroth I anastomosis) or a gastrojejunostomy (Billroth II or Roux-en-Y anastomosis).
Gastric Wedge Resection, Partial, Sub-total and Total Gastrectomy
Indications, Extent of Gastric Resection, Lymphadenectomy, and Reconstructions
Gastric neoplasms represent the most common indication for gastric resection. Masses in the stomach typically fall into one of three groups: gastric adenocarcinoma, gastrointestinal stromal tumor (GIST) and lymphoma. While lymphoma is typically treated non-operatively, both GIST and adenocarcinoma are indications for gastric resection. The majority of patients with gastric masses will present with epigastric fullness, dyspepsia, pain or bleeding. The evaluation of a gastric mass can include cross-sectional imaging with computed tomography, endoscopy with cold forceps biopsy, and/or endoscopic ultrasound with fine needle aspiration (FNA) biopsy [7].
The extent of gastric resection and type of lymphadenectomy should be chosen based on individual patient and tumor characteristics. Wedge resection is reserved for selected GIST that can be completely excised without a formal resection, partial gastrectomy can be used for GIST or rare complications from PUD and subtotal or total gastrectomies are the preferred options for patients with gastric adenocarcinoma.
GISTs are rare tumors that are typically located in the stomach. These tumors arise from the cells of Cajal which are part of the autonomic nervous system of the stomach. These tumors express Kit protein, a tyrosine kinase receptor, and many express CD34. Diagnosis is confirmed with FNA. Surgery is indicated with goal of a grossly negative margin, and non-anatomical wedge resections are usually employed [8]. Care is taken to not to spill tumor contents during resection out of concern for seeding the peritoneal cavity. GISTs usually do not spread via the lymph system, therefore lymphadenectomy is not indicated. Imatinib mesylate is commonly used as neoadjuvant therapy from 3 to 6 months to decrease tumor size and/or allow for wedge or complete resections. Imatinib is also used as adjuvant therapy after surgery in many cases.
The incidence of gastric adenocarcinoma has declined in the past century. Epidemiologic studies have linked this disease to a diet high in nitrates, lower socioeconomic status, Helicobacter pylori, chronic atrophic gastritis and Asian heritage. Gastric adenocarcinoma diagnosis is confirmed with endoscopic biopsy, and patients should be evaluated for precise location and extent of the tumor and metastatic disease with computed tomography of the chest and abdomen. If no evidence of metastatic disease is found, endoscopic ultrasonography should be performed to assess depth of invasion and to evaluate regional lymph node basins. Should involvement of the aorta, vena cava or celiac axis be identified the tumor is considered unresectable [7].
The extent of gastric resection is chosen based on tumor location. A sub-total gastrectomy is usually chosen for patients with tumors below the incisura angularis and at least 6 cm from the GE junction. A total gastrectomy is typically needed for patients with tumor above the incisura angularis, in the gastric body or fundus or less than 6 cm from the GE junction.
Gastric adenocarcinoma readily metastasizes via the lymph system and lymphadenectomy is recommended during surgical resection with curative intent. The extent of lymphadenectomy has been controversial for many years, and early data from studies performed in western countries (the Dutch D1D2 gastric cancer trial [9] and the MRC Trial [10]) showed that a more limited lymphadenectomy (D1) was associated with improved peri-operative outcomes and similar oncologic outcomes. However since that time, other studies and also a 15 year follow up of the Dutch D1D2 Trial have demonstrated a benefit to accurate staging and also to survival with a D2 lymph node dissection [11–14]. A D2 lymph nodal dissection involves omentectomy with clearance of the peri-gastric, hepatic artery, porta-hepatis, splenic, right gastroepiploic, sub-pyloric and base of left gastric artery/celiac lymph nodes. The addition of splenic hilum nodes (level 10), splenectomy and/or distal pancreatectomy, or a peri-aortic lymph nodal dissection (D3 lymph node dissection) has been shown to increase peri-operative morbidity without improving survival [15]. As such, most centers specialized in gastric cancer treatment do advocate for a complete D2 lymphadenectomy (without level 10, splenectomy and/or distal pancreatectomy) when performing gastrectomy for cancer with curative intent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








