Oxygen molecules diffuse across the membrane from the sample gas into the sensor, and are reduced to hydroxyl ions at the cathode. The hydroxyl ions then migrate to and oxidise the lead anode to form lead oxide. These reactions create a flow of electrons, which can be measured. The lead anode is slowly consumed by the oxidation reactions, which limit the lifespan of the fuel cell.
The polarographic (Clark electrode) analyser functions in a similar manner to the fuel cell. It consists of a noble cathode (usually platinum) and a silver/silver chloride anode contained within a potassium chloride electrolyte solution. In contrast to the fuel cell, an external voltage of 0.6 V is required to cause the reduction of oxygen at the cathode. A Teflon membrane separates the cell from the gas sample. 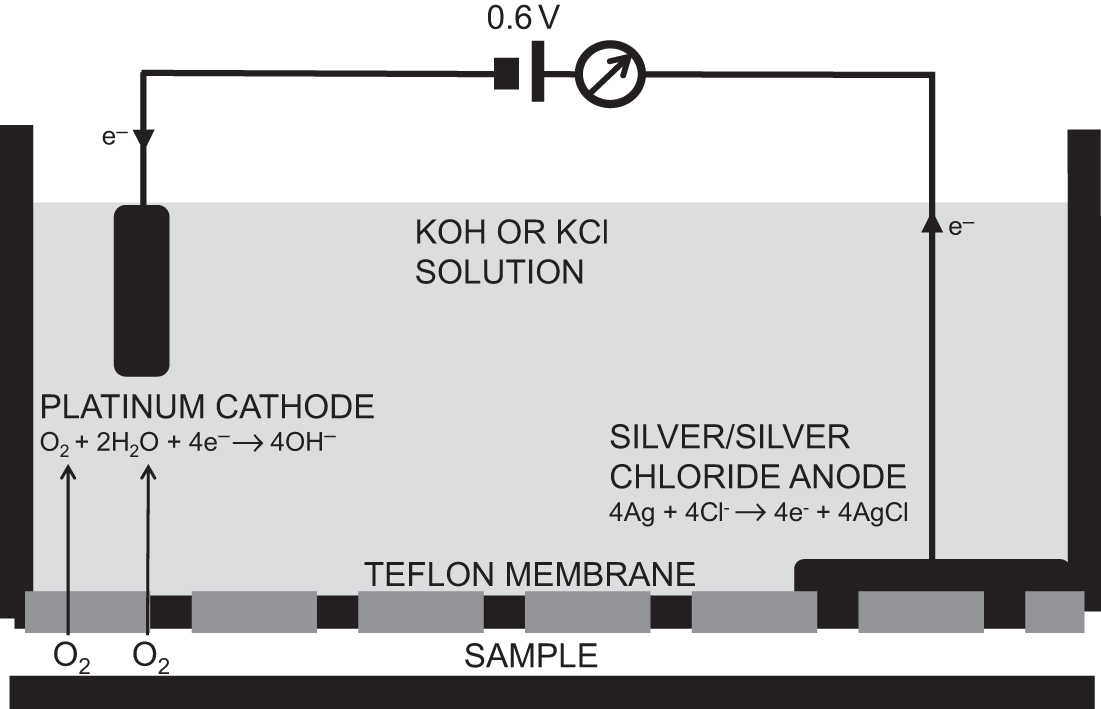
Oxygen diffuses across the Teflon membrane and the electrolyte solution to contact the polarised platinum cathode, where it is reduced to hydroxyl ions. At the silver/silver chloride anode, the silver is oxidised to silver chloride in a reaction that releases electrons.
As the chemical reactions occur, an electric current that is directly proportional to the partial pressure of oxygen will flow between the anode and the cathode. An amplifier can be used to increase the signal output.
The Clark electrode has a faster response time than the fuel cell because of its external power supply. The solubility of oxygen, similar to all gases, increases as temperature decreases. Thermistors are sometimes used in these devices to compensate for the varying amounts of dissolved oxygen at different temperatures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





