FIGURE 14.1 Deep brain nuclei: Targets for brain electrodes.
1. Parkinson’s disease. Parkinson’s disease is a neurodegenerative condition due to loss of dopaminergic cells in the substantia nigra. Dopamine inhibits the rate of firing of the neurons that control the extrapyramidal motor system. Depletion of dopamine results in diminished inhibition of these neurons and unopposed stimulation by acetylcholine. Diagnosis is clinical and based on cardinal symptoms of resting tremor, bradykinesia, rigidity, and gait disturbance. Treatment is designed to increase the concentration of dopamine in the basal ganglia or to decrease the neuronal effects of acetylcholine. Replacement therapy with the dopamine precursor levodopa is the standard medical treatment but is associated with numerous side effects including dyskinesia. Bilateral DBS of the STN is the most commonly performed procedure for surgical treatment of advanced Parkinson’s disease, and when successful, it results in reduced motor disability, reduced motor fluctuations, and a reduction in levodopa-induced dyskinesia. Medication dosages can be lowered postsurgically, with a resultant reduction in medication-induced adverse effects. DBS can add value to the best medical treatment and it is a cost-effective intervention, although it is not curative of Parkinson’s disease and it does not alter the progression of disease [5]. Optimal outcome primarily depends on patient selection and accuracy of lead placement in the proximity of the target nucleus. Patient selection is done by a multidisciplinary team. Patients are selected on the basis that they respond to dopaminergic medications, exhibit medically refractory motor fluctuations or dyskinesia, and they do not have significant cognitive decline or depression.
2. Dystonia. Dystonia is a clinical syndrome characterized by muscle contractions causing abnormal repetitive and twisting movements resulting in painful and debilitating postures. There is no medical cure, and medical therapy is aimed at symptom relief. Stimulation of the globus pallidus internus (GPi) has been shown to reduce symptoms and significantly improve the quality of life of these patients [6].
3. Essential tremor. Essential tremor is the most common form of pathologic tremor. It affects mainly the hands, head, voice, and tongue but lower extremities can also be involved. Essential tremors can be treated with DBS of the ventral intermediate thalamus [4,7].
B. Other indications. Indications of DBS have now expanded and include many other neurologic and psychiatric conditions. For each specific disease, such as Tourette’s syndrome, cluster headaches, chronic pain and Alzheimer’s disease, a specific target is developed [4,8,9]. Severe depression and obsessive–compulsive disorder have been treated with stimulation of the subcallosal cingulate gyrus, the anterior limb of the internal capsule, or the nucleus accumbens [10]. Some studies have shown positive effects of anterior thalamus stimulation for refractory epilepsy (Table 14.1) [11,12].
Table 14.1 Indications for deep brain stimulation

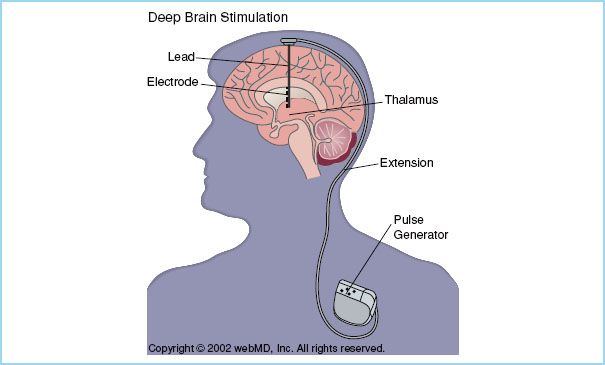
FIGURE 14.2 An electrode is placed in the vicinity of a deep brain nucleus and via a subcutaneous lead is connected to a pulse generator which has been inserted in the subclavian space. Two electrodes can be inserted into one generator and still operate independently. (Reproduced with permission)
III. The procedure. Through a burr hole, a stimulating electrode is placed within the vicinity of a deep brain nucleus using brain mapping and fluoroscopy. Following confirmation of placement, this electrode is connected to a generator inserted in the subclavian space using a subcutaneous lead (Fig. 14.2). The pulse generator produces high-frequency electrical stimulation. Electrical stimulation of the target nucleus is programmed to obtain the best symptom relief and to allow for flexibility and potential long-term symptom control. In Parkinson’s disease, bilateral electrodes are usually inserted and controlled separately with one single pulse generator. Target nuclei are localized using a combination of methods. First, magnetic resonance imaging (MRI) is used to locate the nucleus and identify stereotactic coordinates. STN and GPi are visualized on MRI, however, the thalamic nucleus is usually not visible on standard MRI and target localization is instead performed based on proportional system coordinates or stereotactic superimposition of standardized brain atlases. Intraoperatively, target location is confirmed by electrophysiologic monitoring. Each nucleus has a characteristic neural firing profile allowing its identification. An electrode is inserted 10 to 15 mm above the target nucleus and then it is advanced in steps of 0.5 to 1 mm while spontaneous neuronal discharges are recorded (microelectrode recordings [MER]) until the electrode is in close proximity to the target. A stimulation trial (macrostimulation) allows for assessment of clinical improvement and side effects such as dysarthria, dyskinesia, sensory deficits, eye movement, and muscle cramps. This last step requires an awake, cooperative patient. Anesthetic agents may reduce the quality of MER although some studies have reported good quality of MER of the STN under general anesthesia [5,13]. Macrostimulation may be difficult to interpret if the patient is fatigued.
A. Description of surgery. On the day of surgery, a rigid frame is fixed on the patient’s head following a scalp block or local anesthetic infiltration at the pin insertion sites. Local anesthetics most commonly used include bupivacaine 0.5%, ropivacaine 1% alone or in combination with lidocaine 1%. A blend of mepivacaine 1% and tetracaine 0.2% is used in one of the authors’ institutions. Tetracaine, is a long-acting ester-type local anesthetic. In addition, small sedative doses of propofol can be given at the time of head fixation with the stereotactic frame. With the frame in place, MRI is performed to define the target nucleus. Images are used to map and localize the area of interest and to plan the path for the introduction of the electrode. The patient is then transferred to the operating room and placed in a semi-sitting position for burr hole and electrode placement. After the position of the electrode is confirmed, a final quadripolar electrode is inserted and sutured in place. If bilateral electrodes are required, all the steps are then repeated on the contralateral side. At the end of the procedure or on a subsequent day depending on local practices, electrodes are internalized, the leads are tunneled and the pulse generator is inserted in a subcutaneous pocket below the clavicle. This second stage of the procedure is almost always done under general anesthesia. The implanted electrode remains inactive during this second stage so choice of anesthetic technique will be influenced by patient factors and the presence of comorbidities [8,14].
CLINICAL PEARL
While many patients tolerate frame placement and MRI under local anesthesia with minimal sedation, some patients including those with severe cervical dystonia or with uncontrolled body and limb movements, and children will require moderate sedation or general anesthesia.
B. Anesthesia. The anesthesiologist faces many challenges during a DBS procedure. The anesthetic goals are to provide maximum patient comfort with minimal interference with intraoperative MER and macrostimulation. The procedure is relatively long in duration, usually several hours, and most patients who present for surgery are disabled as a consequence of their neurologic condition.
The anesthesiologist needs to be prepared to rapidly diagnose and treat any intraoperative complications. Choice of the most appropriate anesthetic modalities has been widely discussed in the literature because of the possible interference with MER, by altering neuronal firing frequency. It is not known to what extent anesthetic drugs influence the MER as the effect depends on the individual agent and the intended target. Controversy is compounded by the fact that anesthetic management differs among centers and ranges from local anesthesia alone, or in combination with supplemental sedation, to the use of general anesthesia for every case.
1. Local anesthesia. The traditional method is to use only local anesthetic for burr hole placement and keep the patient unsedated throughout the procedure.
2. Monitored anesthesia care. The common approach; however, is the use of monitored anesthesia care with sedation when patient cooperation is not needed. Propofol sedation should be stopped approximately 15 to 20 minutes before neurophysiologic mapping to allow dissipation of drug effects. Propofol infusion, 25 to 75 μg/kg/min alone, or in combination with remifentanil infusion 0.02 to 0.05 μg/kg/min is commonly used [2,3]. The pharmacokinetic behavior of propofol in patients with Parkinson’s disease may differ from the general population and its sedative effect may be more profound [8]. Close monitoring with frequent adjustment of infusion rates may be necessary. Dexmedetomidine, an α-2 receptor agonist, administered at a dose of 0.3 to 0.6 μg/kg/h, may be the drug of choice, as it provides sedation with minimal respiratory depression, and has little or no effect on electrophysiologic mapping and functional testing. Dexmedetomidine also decreases the intraoperative use of antihypertensive medications. Hypotension and bradycardia, however may occur with dexmedetomidine [15]. Oversedation can still lead to upper airway obstruction due to relaxed muscle tone. Some patients, especially the older, are more sensitive to the effects of dexmedetomidine and the risks of oversedation and longer recovery times need to be carefully addressed. It might be necessary to reduce the infusion rate of dexmedetomidine after a couple of hours in order to maintain the same level of sedation [15]. Benzodiazepines are not used because they can abolish MER, increase the threshold of stimulation and may induce dyskinesia [3]. It can be difficult to predict a patient’s response to sedation during brain surgery as every patient responds differently. The key is careful and gentle titration of sedative(s). And in some cases, no sedation may be the safest option.
CLINICAL PEARL
Anesthesia agents may interfere with microelectrode recordings and therefore sedation should be discontinued or decreased to a minimum during recordings and stimulation.
3. General anesthesia. General anesthesia may be the only option for patients with severe diseases and uncontrolled movements or for pediatric patients. The advantages of using general anesthesia include reduced anxiety, reduced neck and back pain, and reduced painful dystonia in patients. It has been reported that general anesthesia can produce surgical outcomes similar to that found using local anesthesia alone [5,13,16]. However, a recent report showed comparable long-term motor outcome between general anesthesia and local anesthesia but significant cognitive decline and higher stimulation side effects in the general anesthesia group [17]. In these studies, adequate MER were obtained with all agents: propofol, dexmedetomidine, remifentanil, and volatile agents. It should be noted that DBS surgery can be done without MER. MRI-guided only DBS surgery under general anesthesia or local anesthesia may be a viable option [16]. The use of depth of anesthesia monitors such as bispectral index (BIS) has been studied to help titrate sedation, or anesthesia depth during recordings. However, the reliability of BIS has not yet been proven and conflicting results have emerged [18,19].
4. Specific anesthetic considerations (Table 14.2)
Table 14.2 Anesthetic considerations for deep brain stimulation

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







