Pregnancy
Severe skeletal deformities
Severe obesity
Aortic or renal artery aneurysm?
Urinary obstruction distal to stone
Uncontrolled anticoagulation
Uncontrolled hypertension
Uncontrolled urinary tract infection
Procedure Techniques
Classically, PCNL is done with the patient in a prone position. Whether an infracostal or supracostal approach is taken depends on both the location of the kidney relative to the ribs as well as the position of the stone within the kidney. Renal access is obtained by the insertion under fluoroscopic or ultrasonic guidance of a needle to the stone site. A wire is then advanced through the needle and into the collecting system. The needle is removed over the wire and an incision along the wire is made to allow insertion and removal of successively larger dilators. Once the tract is suitably dilated, a sheath is placed over the last dilator, which is then removed. Next, a rigid nephroscope is placed through the sheath. Once the nephroscope is in place, a variety of lithotripters (ultrasonic, pneumatic, electrohydraulic, and laser) exist that are designed to be inserted through the nephroscope channel where they can then be visually directed to treat the stones and observe the surgical progress. An irrigation solution, also attached to the nephroscope, is used to flush stone fragments from the kidney and out of the nephroscope and to maintain a functional visual field. This is either a passive, gravity-driven system where the height of the irrigation bag determines the irrigation fluid pressure or the fluid is connected to a pump that automatically adjusts flow to maintain the programmed pressure. The duration of the surgery (irrigation time) depends upon the complexity of the disease, stone number, and their locations, but a typical procedure with uncomplicated disease can be expected to last 2–3 h. After completing the PCNL treatment, a catheter is placed into the pelvis to serve as a nephrostomy tube. If stones are large or difficult to treat, not only will the operative time increase, but additional procedures may be required [3, 6].
Over the past 35 years, the PCNL procedure described above has seen the introduction of several innovations. Most notable are the use of the supine position, tubeless PCNL, and the so-called miniperc technique.
Supine Position: PCNL in the supine position is safe [7–10]. The primary advantages of surgery in this position are that the complications related to prone positioning are avoided, most prominently difficulty with intraoperative ventilation for obese patients. Unfortunately, supine positioning can promote collapse of the collecting system, a smaller surgical field, and increased difficulty in upper-pole calyceal puncture [7].
Tubeless PCNL: Tubeless PCNL, which can be performed with a variety of techniques, was first introduced in 1997 [11]. At the end of the procedure, an internal ureteral stent or ureteral catheter is placed in most cases, but occasionally no drainage is placed at all [12]. The reported benefits of tubeless PCNL include fewer complications, lower cost, shorter hospital stay, lower analgesia requirements, and a quicker return to normal activities [11–14]. Hydrothorax, pseudoaneurysm, arteriovenous fistulae, bleeding, and splenic injury are the reported major complications from tubeless PCNL [12].
Mini-percutaneous Nephrolithotomy (Miniperc): This procedure, introduced in 1988, employs a smaller working sheath and nephroscope. Development of the miniperc technique stemmed from an effort to introduce PCNL to the pediatric population. Performed through a 13F Amplatz Sheath®, the hope was that procedure-related morbidity would be reduced [14]. However, clinical studies of the miniperc procedure have failed to demonstrate decreased perioperative morbidity or improvement of perioperative pain management [15].
Anesthetic Considerations in PCNL
Commonly, general anesthesia with an endotracheal intubation is preferred for PCNL, although local anesthesia plus sedation and spinal anesthesia have also been successful [16, 17].
Regardless of the anesthetic technique used, a thorough preoperative evaluation should be done for all patients prior to PCNL. This assessment includes not only the customary history and physical, but attention must also be paid specifically to eliciting whether an existing urinary tract infection exists, if it has been treated, whether the patient is receiving anticoagulants, and the plan for perioperative antibiotic coverage. Active urinary tract infection (UTI) and uncorrected bleeding diathesis are relative contraindications for the procedure. All anticoagulation medications including aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) are typically held for 5–7 days prior to surgery. Patients who are taking anticoagulation medications should have suitable competence of coagulation established. Patients who are receiving antibiotic treatment for UTI should have a negative urinary test before surgery. Laboratory tests should focus on the issues revealed by the preoperative evaluation. In general, blood type and screen are recommended for the patients who are at high risk of intraoperative bleeding. Some of the bleeding risk factors are listed below. A preoperative discussion with the urologist will help clarify the surgical issues, confirm the risk status, and elucidate proper selection, timing, and dose of antibiotic.
Nephrostography or retrograde ureteropyelography (RPG) is often used to identify the renal and upper urinary tract structure and locate the obstructions during PCNL. When radiographic iodinated contrast media (ICM) is used during any procedure, ICM-induced adverse reactions are possible. A previous adverse reaction to ICM, a history of asthma and atopy, dehydration, renal disease, and extremes of age are predisposing factors for developing a reaction to ICM [18]. However, ICM injected into the urinary tract is generally believed to present a far lower risk of adverse reaction than from intravenous injection.
ICM is often classified as ionic versus nonionic and high osmolality versus low osmolality. One large Japanese case series of intravenous use of ICM revealed that the overall risk of adverse reaction was 12.7% with ionic ICM and 3.13% with nonionic ICM. Severe adverse reaction to ionic ICM had an incidence of 0.2%, fivefold the risk of 0.04% from nonionic ICM [19]. A meta-analysis specifically addressing this question also found a higher risk of severe reactions with ionic ICM [20].
Symptoms of adverse reactions may develop within 1 h after ICM administration (immediate reactions) or several hours to several days after administration of ICM. Controversy exists regarding prophylaxis and treatment for the adverse reactions to ICM.
Beyond typical anesthetic concerns, additional consideration must be given to the physiological changes and common complications associated with the PCNL. Kidneys are retroperitoneal organs. The right kidney lies adjacent to the 12th rib. The liver, duodenum, and the hepatic flexure of the colon are all located in close proximity. The left kidney lies a bit more superiorly, near the 11th and 12th ribs, with pancreas, spleen, and the flexure of colon nearby. Bilaterally, the kidneys are also in close proximity to the pleura. Most commonly reported organ injuries are due to these anatomic relationships. The major complications during PCNL include bleeding, bowel and collecting system injury, traumatic arteriovenous fistula or false aneurysm, sepsis, atelectasis, pneumothorax, pleural effusion, and hemothorax [21, 22]. Even tetraplegia, presumably from a rare air embolus, is possible (personal communication; D Hegland, MD 1/20/2012). Munver et al. reported that the overall complication of PCNL was 8.3%. Compared to a complication rate of 4.5% during procedures approached with subcostal access, the rate of complication in supracostal access cases was 16.3% [23]. The operative side also appears to influence overall procedural risk. Hopper et al. found that the right kidney had a higher rate of intrathoracic complications than the left kidney, 29% versus 14%, respectively [24].
Although some complications are readily identified during the procedure, many signs and symptoms are slow to develop, making early diagnosis and treatment difficult. For instance, large irrigation volume obscures blood loss estimation and may delay recognition of hypovolemia, when beta-blockers and vasoconstrictors might also be in use. Shivering and high fever are obscured by general anesthesia with muscle relaxants and might not become evident until the patient has emerged from anesthesia. Hypotension from sepsis may be interpreted as hemorrhagic hypovolemia. Signs of pleural injury and pneumothorax can be subtle – never reaching the drama of a tension pneumothorax. Maintaining a degree of suspicion and close observation of the patient for the signs of respiratory distress and splinting postoperatively are important for prompt recognition and management.
Perioperative Infection Control and Sepsis Prevention: UTI plays an important role in urinary stone disease. A bacteremia rate of 15% and a bacteriuria rate of up to 35% can be expected following PCNL [25]. All patients who undergo PCNL should have prophylactic antimicrobial treatment. The American Urological Association (AUA) guidelines currently recommend prophylactic perioperative coverage consisting of either a cephalosporin (first/second generation), aminoglycoside, or metronidazole. Ampicillin/sulbactam and a fluoroquinolone are alternative therapies. Duration and doses of antibiotics vary but in general, a single dose is sufficient for prophylaxis. Absent an indication for continued treatment, the prophylactic antimicrobial therapy should be discontinued within 24 h. With the exception of fluoroquinolones and vancomycin (which should be infused slowly over 60 min prior to incision), all antibiotics should be given within 60 min prior to skin incision [26, 27].
Bleeding and Blood Transfusion: For the patients who have the indications of bleeding diathesis and liver disease, the coagulation profile should be evaluated prior to surgery. Medications affecting coagulation including NSAIDs and aspirin are often discontinued from 1 to 2 weeks prior to surgery. For the patient who takes antiplatelet medication(s) for cardiac protection, a cardiology consultation regarding perioperative management of anticoagulant therapy is recommended prior to discontinuing these medications. The most common bleeding is from a venous source from within the tract [28–30]. Placement of a large nephrostomy tube or clamping of the nephrostomy catheter will affect a tamponade, facilitate clot formation, and arrest of the hemorrhage. Occasionally, the bleeding is not amenable to these maneuvers, and angiography with embolization is necessary. Overall the rate of reported blood loss requiring transfusion is 0.4–23% [29–32]; the average decrease in hemoglobin for single puncture PCNL reported by Stoller ML et al. was 2.8 g/dL [33]. The risk of sustaining significant hemorrhage is increased by diabetes, obesity, multiple-tract procedures, prolonged operative time, and intraoperative complications [30, 31]. Should these risk factors exist, closer observation of the patient’s hemodynamic status is warranted throughout the perioperative period. Hemoglobin and hematocrit laboratory results must be considered in the context of preoperative values, estimated intraoperative blood loss, irrigation fluid volume and pressure used, and surgical duration because of a concern that the absorption of irrigation fluid and the intravenous fluid administered may obscure the diagnosis. Blood transfusion decisions ultimately will be guided by the entire clinical course and the current picture.
Supracostal Approach-Related Complications: The supracostal approach to PCNL is understood to have an increased risk of adjacent organ injury, especially for intrathoracic complications, when compared to the subcostal approach. Complications from supracostal PCNL include pneumothorax, hydrothorax/hemothorax, vascular injury, pleural effusion, nephron-pleural fistula, increased risk of intraoperative bleeding, and increased postoperative pain [34, 35]. Most of the symptoms stemming from pleural injury are subtle and may not be appreciated during the procedure. Consequently, one must remain vigilant to changes in peak airway pressure and oxygenation. Regular communication with the surgeon regarding the progress or difficulties with the operation also promotes earlier awareness and allows prompt treatment. Postoperatively, in addition to routine monitoring of respiratory status and oxygenation saturation, all patients who have undergone supracostal access for PCNL should also receive a chest X-ray to exclude intrathoracic injury.
Positioning-Related Issues: Prone position is the most common patient position for PCNL. Typically, the patient is induced and the trachea intubated on a transport bed (stretcher), and the patient is then rolled into the prone position on the operating table. For patients in whom an adequate imaging study cannot be obtained or in whom intravenous iodinated contrast dye is contraindicated, a retrograde pyelography precedes renal access. In such cases, a lithotomy position for retrograde pyelography and placement of ureteral injection catheter will be the initial position, before being turned prone.
The prone position alone is associated with a variety of position-related complications. To avoid cervical spine injury during positioning, the head should be held in a neutral position through the turn and positioning. The head is usually placed into a foam cushion that allows it to rest in a downward facing neutral position. Eyes, nose, and ears should be confirmed to be free from pressure. Neck extension or head rotation could also impede carotid and/or vertebral artery blood flow and venous return. Appropriate padding protects pressure points and allows the viscera to hang in a dependent position, which serves to decrease intra-abdominal pressure. Pulmonary compliance is improved and ventilation-perfusion mismatch decreased. Increased intra-abdominal pressure impedes venous return and decreases cardiac output with an average decrease in cardiac index of 24% [36]. Obstruction to inferior vena cava (IVC) blood flow causes venous engorgement upstream of obstruction and aggravates surgical site bleeding.
The etiology of peripheral nerve injury is usually multifactorial, requiring both a direct pressure and stretch component. When hypotension or anemia is superimposed, the magnitude of the pressure and stretch needed to cause injury is lessened. Neural structures experiencing any pressure or stretch ranging from the eyes, brachial plexus, ulnar nerve, to common peroneal nerve are at risk.
The effects of irrigation: The large volume of irrigation fluid used during PCNL can decrease body temperature. Hence, monitoring core temperature is routine. Even when convective body warming and warmed intravenous fluids were administered, Rozentsveig et al. reported that the esophageal temperature still decreased from a mean baseline value of 36.4 °C to 35.2 °C during PCNL [37]. The importance of incorporating an appropriate irrigation fluid warmer in addition to the other patient warming aids in more common use during PCNL is underscored. Currently, fluid absorption during PCNL has not been identified as a significant risk. There is insufficient evidence to support that fluid absorption confers any significant clinical effects on blood pressure, heart rate, electrolyte balance, and metabolic changes [38, 39]. Nevertheless, caution should still be exercised for patients who will not tolerate additional fluid as some absorption of irrigant is likely.
Anesthesia Considerations in ESWL
While PCNL remains a common method for treating urinary tract stone disease, with the development of extracorporeal shock wave lithotripsy (ESWL), it has been supplanted as the primary treatment modality for most patients. ESWL was introduced into clinical practice in 1980 [40] and is now the first-line surgical treatment for about 90% of kidney and ureteral stone disease [6, 41, 42]. As with PCNL, treatment recommendations vary based on location, composition, and size of the stones (Fig. 5.1) [6, 43–45], but convincing evidence suggests that the vast majority of renal stones, with only a few exceptions (Table 5.2) [46, 47], can be adequately addressed by ESWL [48].
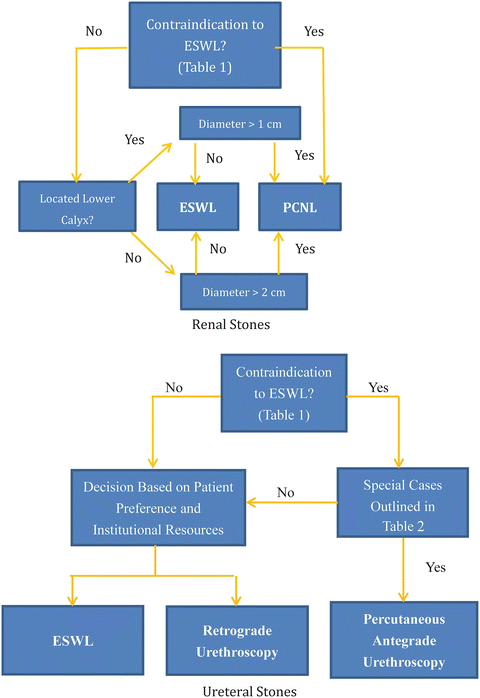

Fig. 5.1
Typical management paradigms for renal and ureteral stones
Table 5.2
Conditions when management with ESWL is uncommon
Large, impacted upper ureteral stones |
Concomitant renal stones |
Previous urinary diversion |
Presence of renal transplant |
Fundamentals of Lithotripsy
ESWL fractures stones into small fragments which can be passed in the urine. This effect is accomplished by targeting and then focusing shock waves on the small volume occupied by the stone. Ultimately, the energy of the waves overcomes the tensile strength of the stone. Various mechanisms by which the energy of the shock waves produces stone fragmentation have been postulated. Compressive and tensile forces as well as cavitation from rapid expansion and dissolution of gas bubbles are likely the predominant mechanisms. Effective lithotripsy relies on transmission of relatively unattenuated shock waves through the water density of the tissues until they arrive at the focal point, aimed to be coincident with the different density of the stone.
Lithotripters must accomplish four functions:
1.
Precisely locate the stone target.
2.
Generate powerful acoustical shock waves.
3.
Project and focus the shock waves on the small volume occupied by the target.
4.
Couple the generator to the patient.
Fluoroscopy and ultrasonography are used to visualize and target the stone and to determine the focal point of the shock waves. These modalities allow the proceduralist to follow the progress of treatment and to make appropriate adjustments to power, shock wave delivery rate, and aim. Fluoroscopy was utilized in the first-generation systems. It is excellent at detecting radiopaque stones. It can also be used in conjunction with the introduction of contrast media for visualizing radiolucent stones. Ultrasonography, which was introduced with second-generation machines, can visualize all types of stones without the allergic or nephrotoxicity risk associated with the use of intravenous contrast. Additionally, ultrasonography has the advantage of being a more economical system, allowing continuous real-time imaging and, of course, eliminates any risk of the patient or provider receiving any ionizing radiation. Acoustic imaging with ultrasound has predictable technical limitations including poor visualization in obese patients or when the stone is obscured by air-filled loops of bowel. It is also less effective at visualizing small stones. Many newer systems allow the proceduralist to use either modality, switching between the two as desired.
The original lithotripter (Dornier HM3®) utilized an electrohydraulic shock wave generator. In this system, high-voltage electrical current is passed through an electrode (known as a “spark gap”) which is placed in a water bath. When the spark gap fires, it causes a gas bubble to form by vaporization. Rapid expansion and collapse of the bubble generate the therapeutic pressure waves. Later generations of lithotripters utilized arrays of piezoelectric crystals to generate the pressure wave. Electromagnetic generators utilize an electromagnetic field to create vibration in a metallic membrane. The membrane then creates acoustic pressure waves.
Acoustical waves must be focused on a relatively small focal point to deliver sufficient energy to fragment a stone. The original first-generation systems focused the electrohydraulically generated wave by means of a metal ellipsoid. Piezoelectrical systems focus the waves through the hemispheric orientation of the crystal array. Electromagnetic systems rely on a cylindrical reflector or acoustic lens to focus the waves. The newer systems allow a tighter focusing of the waves. More tightly focused waves deliver higher energy to a smaller treatment volume, offer shorter treatment times, and lead to less signal attenuation in nontarget tissues.
As the shock wave is propagated through tissue planes, energy is lost (transferred) at every interface where the wave enters a tissue with a different density. As the human body is near water density, water is commonly used as a coupling media to deliver, with the least attenuation, the most energy into the body. The original Dornier HM3® model involved placing the patient in a water bath to couple the acoustic wave to the patient. The obvious safety concerns involved with immersing a patient to the neck under general anesthesia, so that a high-energy shock wave originating underwater will couple to the patient, have relegated this technique to a historical note. Second- and later-generation systems utilize water-filled cones or cushions as well as silicone membranes and/or gel to allow an air-free coupling of the wave to the patient.
Anesthetic Considerations
As the ESWL acoustic waves travel to the target focal point, any anatomical structure passed may experience shock wave-induced stress or cavitation including skin, lumbar muscles, periosteum of rib or vertebrae, and the renal capsule [49–51]. Treatment parameters such as applied voltage, focal point pressure, and volume of treated tissue affect the experience of pain [52]. Early ESWL treatments were so painful they required either neuraxial or general anesthesia. ESWL manufacturers have developed machines with lower applied voltage and markedly decreased focal point volume. These changes maintain similar or only modestly decreased focal point pressures while dramatically reducing energy density. This reduction in energy translates to less pain, allowing most treatments to proceed with sedation and analgesic regimens and, notably, to be performed on ambulatory patients in an outpatient setting [53]. Unfortunately, these newer systems may result in higher retreatment rates [54].
Numerous attempts have been made to block the cutaneous discomfort of ESWL treatment. Local anesthetic infiltration [55], intercostal nerve blocks [56], and topically applied lidocaine/prilocaine mixtures (EMLA® cream, Astra Pharmaceuticals Products Inc., Westborough, MA, USA) have all been used. Although these techniques decreased cutaneous pain at lower energy settings, they did not seem to influence sedative or narcotic requirements at higher treatment energies [50, 57–60].
Modern anesthetic management of routine ESWL treatments on adults has centered on providing effective sedative/analgesic regimens. Various techniques including meperidine and promethazine [52], midazolam with alfentanil [61], fentanyl [62], and ketamine have all been successfully used. Considerable research on the use of alfentanil by various routes (physician-controlled infusion, patient-controlled analgesic (PCA), and pharmacokinetically based target-controlled infusions) has shown this drug to be very effective [49, 53, 62].
More recent studies have looked at propofol or dexmedetomidine in combination with fentanyl, morphine, or ketamine [63–65]. All have been effective, but in a comparative study [66], dexmedetomidine with low-dose fentanyl yielded better pain relief as measured by Visual Analog Scale. The dexmedetomidine group also had higher oxygen saturation (SpO2) and a lower respiratory rate. The authors suspected (although they did not actually measure) that tidal volume was larger in the dexmedetomidine group, a change that could lead to greater stone excursions with respiration and make treatment more difficult. Interestingly, no score of the proceduralist’s satisfaction with the adequacy of sedation or operative conditions was reported in this study.
The movement of stones during treatment is undesirable because it moves the target from the focal point where the shock waves are strongest. The two consequences are that treatment time will be prolonged as shock wave delivery is suspended until the stone returns to the treatment focal zone or, if shocks are not interrupted, the tissue coming into the focal zone will receive that energy and may become injured. Spontaneous ventilation has been shown to displace stones over 12 mm. Even such small movements can increase treatment times [67]. Various measures can decrease stone excursion. During procedures performed with spontaneous ventilation, adequate sedation can decrease stone excursion to about 5 mm [66]. Abdominal binders have also been shown to reduce stone excursions [68].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







