KEY POINTS
Extracorporeal membrane oxygenation (ECMO) can be used to provide support to selected patients with severe acute respiratory failure and severe hypoxemia.
The two major ECMO modalities are veno-venous (VV) and veno-arterial (VA), but most cases of extracorporeal lung support use VV-ECMO.
The prospective, randomized Adult ECMO study (CESAR trial) reported a 31% improved outcome in patients transported to a specialized center for possible ECMO (63% vs 47% survival without disability; relative risk 6-month death or severe disability 0.69, 95% CI 0.05-0.97; RR death 0.73, 95% CI 0.52-1.03).
Significant adverse events and complications can occur during ECMO, most related to hemorrhage, but are becoming less common with improved technology and reduced anticoagulation requirements.
ECMO is used in patients with severe hypoxemia related to ARDS, 2009 Influenza A (H1N1)–associated ARDS, trauma, and pulmonary embolus.
Survival to discharge in adult patients receiving ECMO for respiratory failure is 52% from the Extracorporeal Life Support Organization (ELSO) registry.
VV-ECMO is now being used as a therapeutic option to bridge patients with advanced lung disease to lung transplantation, avoiding the use of mechanical ventilation and allowing aggressive physical rehabilitation.
A new adult ARDS ECMO multicenter clinical trial has been initiated, entitled ECM O to rescue Lung Injury in severe ARDS (EOLIA, Alain Combes MD, Principal Investigator, France).
ECMO is a complex critical care organ support system, and requires an experienced and dedicated team, appropriate equipment, and institutional commitment and leadership.
The current evidence supports the transfer of patients with severe hypoxemia and ARDS to institutions with significant experience in ARDS management and with ECMO capabilities.
EXTRACORPOREAL LUNG SUPPORT
Extracorporeal membrane oxygenation (ECMO) is an advanced treatment option for patients with severe respiratory failure and severe hypoxemia.1-6 The goal of ECMO for lung support is to avoid the use of high levels of oxygen and high airway pressures that may be necessary to support oxygenation and ventilation with mechanical ventilation in severe hypoxemia and acute respiratory failure. Nearly 20% of acute respiratory distress syndrome (ARDS) patients die of severe hypoxemia.7
ARDS is associated with pathologically complex changes in the lung manifested by an early exudative phase followed by proliferative and fibrotic phases.8 The acute inflammatory state leads to increased capillary permeability and accumulation of proteinaceous pulmonary edema, leading to hypoxemia. Hypoxia may further aggravate lung injury, and treatment strategies therefore focus on improvement of oxygenation and correction of the underlying problem.9
Mechanical ventilatory support can be injurious and lead to additional lung injury when used at the extremes of pulmonary physiology, a concept that has been termed ventilator-induced lung injury (VILI).10 There are a number of mechanisms that can lead to the development of VILI, including barotrauma, diffuse alveolar injury due to overdistension (volutrauma), injury due to repeated cycles of recruitment/derecruitment (atelectrauma) and the most subtle form of injury due to the release of local mediators in the lung (biotrauma).11
The goal of ECMO therapy is to minimize VILI while allowing additional time to treat the underlying disease and to permit recovery from acute injury or illness.12 Proper selection of patients for ECMO therefore involves determination of whether the pulmonary disease process is reversible. ECMO for adult respiratory support continues to increase in the United States and worldwide.13
ECMO is a complex critical care organ support system, and requires an experienced and dedicated team, appropriate equipment, and institutional commitment and leadership.14 The current evidence supports the transfer of patients with severe hypoxemia and ARDS to institutions with significant experience in ARDS management, and with ECMO capabilities, to allow further expert evaluation and treatment.15
RESPIRATORY CONDITIONS REQUIRING ECMO
ECMO is used in a number of respiratory conditions that cause acute respiratory failure and severe hypoxemia (see Table 53-1). The most common indication for ECMO for lung support is severe life-threatening hypoxemia associated with inadequate tissue oxygenation, most commonly in patients with severe ARDS. Although oxygenation itself is not clearly predictive of poor outcomes in ARDS, there is increasing evidence that a lower PaO2/FiO2 ratio is predictive of death, especially if the hypoxemia persists over time.16-24
In patients with severe refractory hypoxemia, there is potential utility in the incremental approach to ARDS management (Fig. 53-1), incorporating the use of a number of therapeutic “rescue” strategies for severe hypoxemia.25 Implementation of specific rescue strategies for severe hypoxemia may result in improved oxygenation, improved pulmonary compliance, and ultimately survival in individual patients. There is also the possibility that some of these interventional strategies may have additive effects.
We developed an algorithm for the use of mechanical ventilation and ARDS rescue strategies at the University of Michigan, which begins with the ARDSNet low-tidal-volume management and adds several rescue strategies if the patient remains hypoxemic before ultimately considering ECMO (Fig. 53-2). We have also utilized these treatment strategies in stabilization of patients with severe hypoxemia and ARDS at referring hospitals prior to transport to our institution to enable safer transport.26
It is important to have full knowledge of the evidence base and the results of prospective randomized trials, which have carefully assessed the impact of these treatment strategies on patient outcome in severe hypoxemia and ARDS, but it is important to recognize that large randomized trials are not available for all strategies. Nevertheless, appropriate bedside implementation of these treatment strategies, including ECMO, may provide life-saving salvage in individual patients with refractory hypoxemia due to severe ARDS or other pulmonary conditions.
TYPES OF EXTRACORPOREAL LUNG SUPPORT
ECMO is a pump-driven, veno-venous, or veno-arterial circuit with an oxygenator to provide both oxygenation and ventilation.
Selective CO2 removal can be accomplished with low blood flow rates (10 cc/kg/min) with the device attached with arteriovenous access (arterial cannula inserted into femoral artery, membrane oxygenator with venous cannula return to femoral vein, driving force is patient’s blood pressure).27-31 A potential complication is distal limb ischemia in the limb with the femoral arterial cannula. This technology is effective as an AV-CO2 removal device (effective treatment for status asthmaticus) and allows decrease in minute ventilation to provide more protective lung ventilation and correction of acute respiratory acidosis, but has significant limitations in providing oxygenation support. It is also called pumpless extracorporeal lung assist (PECLA) or interventional lung assist (iLA).32 ILA provides effective CO2 elimination and a modest improvement in oxygenation.
A large cohort study, which included 96 patients with severe ARDS, evaluated the factors determining the efficacy of PECLA and calculated its contributions to gas exchange by monitoring hemodynamic parameters, oxygen consumption, CO2 production, and gas transfer through the device. Within 2 hours of PECLA, PaO2/FiO2 ratio increased significantly, and a fast improvement in arterial CO2 partial pressure and pH was observed in all patients. The PECLA removed 50% of the calculated total CO2 production and rapidly normalized respiratory acidosis. As demonstrated in earlier studies, the patients who were on this therapy were able to be ventilated with a protective lung strategy.
A prospective pilot study of ILA in 51 patients with ARDS documented improved ventilation, decreased plateau pressures with reduced minute ventilation required (see Table 53-2).33 The hospital mortality rate was 49% and adverse events occurred in 11.9% of patients.
| Pre-ILA | 2 Hours After ILA | 24 Hours After ILA | |
|---|---|---|---|
| PaO2/FiO2ratio, mm Hg | 75 (62-130) | 102 (70-127)a | 110 (86-160)a |
| PaCO2, mm Hg | 73 (61-86) | 44 (36-54)a | 41 (34-48)b |
| Arterial pH | 7.23 (7.16-7.30) | 7.38 (7.32-7.46)b | 7.44 (7.37-7.49)b,c |
| iLA flow, L/min | – | 1.8 (1.6-2.0) | 1.7 (1.5-2.0) |
| Pplat, cm H2O | 35 (31-38) | 34 (30-37) | 30 (26-34)b |
| Minute ventilation, L/min | 11.5 (9.3-12.5) | 8.6 (6.4-10.5)b | 6.6 (5.5-8.3)b,d |
Recently, a simple extracorporeal CO2 removal (ECCO2R) device was developed (Decap, Hemodec, Salerno, Italy) that is a modification of a standard continuous VV hemofiltration system equipped with a membrane oxygenator, using a single double-lumen cannula for the venous access. Blood flow is via a nonocclusive roller pump. Blood circulates through a membrane oxygenator then through a hemofilter. The ultrafiltrate from the hemofilter is recirculated into the pre-gas exchanger blood, increasing CO2 removal. There are isolated case reports using this device, and clinical trials are proposed.34-36
In patients who have acute and severe respiratory failure and hypoxemia that fail all advanced modes of mechanical ventilation the use of ECMO is an option. ECMO is a proven modality for treatment of severe respiratory failure in the neonate37,38 and has increased since its inception.39 For infants, pediatric, and adult patients with severe ARDS, ECMO has produced respective survival rates of 85%, 74%, and 52%.40 The indications for ECMO for adult respiratory failure are listed in Table 53-3. Referral to an ECMO center should occur early if there is a suspected need for this technology. This will allow safe transport of the patient and avoidance of the “crash on” with all of its inherent complications.
The technique of ECMO for patients with severe respiratory failure involves a veno-venous or veno-arterial life support circuit with a membrane oxygenator to temporarily take over the functions of the lung. While on ECMO, mechanical ventilator settings are adjusted to minimize VILI and to maximize the recruitment to functional residual capacity with an algorithm that aims to normalize body physiology and minimize barotrauma. This algorithm used in 141 patients with respiratory failure referred for consideration of ECMO yielded a survival rate of 62% in patients with severe ARDS (median initial PaO2/FiO2 ratio of 66).41
Adult Respiratory Failure ECMO Criteria
| Indications | Contraindications |
Duration of Mechanical Ventilation
| There are no absolute contraindications to ECLS, as each patient is considered individually with respect to risks and benefits. There are conditions, however, that are known to be associated with a poor outcome despite ECLS, and can be considered as relative contraindications |
Pulmonary Compliance
| Mechanical ventilation at high settings (FiO2 >0.9, Pplat >30) for 7 days or more |
Oxygenation
| Major immunosuppression (absolute neutrophil count <400/mm3) |
| CNS hemorrhage that is recent or expanding | |
| Contraindication to systemic anticoagulation |
The primary indication for use of ECMO in patients with severe respiratory failure is when the risk of dying from ARDS is considered greater than 80% despite optimal ventilator and medical management. This translates to a PaO2/FiO2 ratio of less than 70 on 100% oxygen.
INITIATION OF ECMO
Once the patient is considered an appropriate candidate for ECMO, the potential risks and complications associated with ECMO should be discussed with the patient’s legal surrogate, and written informed consent should be obtained. We have a template ECMO consent form available on our internal ECMO Web site that is printed in the ICU and then scanned into the patient’s electronic medical record.
When severe ARDS patients are transferred to us for possible ECMO evaluation, we place a right internal jugular and right femoral venous catheter in the event that ECMO is required, so that VV-ECMO cannulation can proceed expeditiously. The ECMO charge specialist is contacted, and an ECMO circuit and ECMO blood pack is prepared.
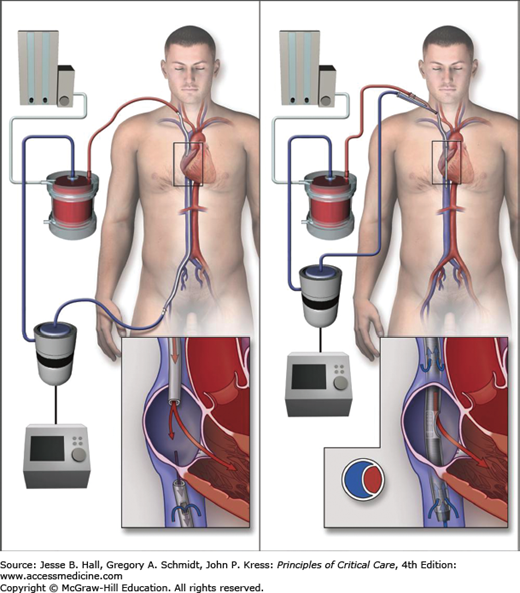
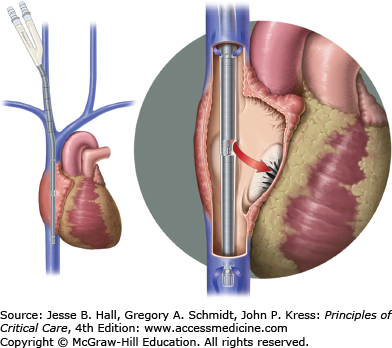
The majority of patients with severe hypoxemia are managed with VV-ECMO. Adult patients are typically cannulated percutaneously with 21 to 23 French catheters for drainage and infusion of blood. Percutaneous venous ECMO cannula insertion is the standard, but surgical cutdown is required in some circumstances. Traditional cannulation for VV-ECMO has been a two-cannula system with venous drainage from the right femoral vein and return to the right atrium via a right internal jugular vein cannula (Fig. 53-3). A single bicaval dual lumen cannula placed in the internal jugular position is preferred if able to be positioned appropriately, since early mobilization of the ICU patient is then feasible. This ECMO cannula allows simultaneous removal of blood from both the superior and inferior vena cavae with return of blood into the right atrium with minimal recirculation.
FIGURE 53-3
Approach to veno-venous ECMO (VV-ECMO) cannulation. Left panel shows the use of two cannulae with (1) venous outflow of deoxygenated blood from the femoral venous cannula and inferior vena cava (IVC) and (2) inflow of oxygenated blood after it passes through the oxygenator where gas exchange takes place into the right internal jugular vein cannula. Right panel shows a bicaval dual-lumen cannula in the internal jugular vein extending through the right atrium with its tip in the IVC. Venous blood is withdrawn from the IVC and reinfusion of oxygenated blood is via a medial port in the right atrium adjacent to the tricuspid valve, delivering oxygenated blood directly into the right ventricle. (Reproduced with permission from Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. November 17, 2011;365(20):1905-1914.)
In most adults, a 27 to −31 French bicaval dual-lumen cannula is percutaneously inserted with a Seldinger technique using an extended length guidewire (0.038 in guidewire, 100 or 210 cm length) to ensure that the distal port tip of the cannula is positioned in the inferior vena cava (Fig. 53-4) for venous drainage to the ECMO circuit with the oxygenator. The proximal drainage port drains blood from the superior vena cava. A uniquely designed medial infusion port returns blood to the right atrium for concentrated oxygen delivery. Optimal orientation of this medial infusion port is critical and we have used fluoroscopy or transesophageal echocardiography in some cases to ensure positioning and adequacy of support (Fig. 53-5).
The size (resistance) of the venous drainage cannula limits the extracorporeal blood flow, therefore placement of the largest cannula possible is ideal. Ultrasound imaging of the vein can assist in providing information regarding the diameter of the patient’s central vein to be cannulated for VV-ECMO.
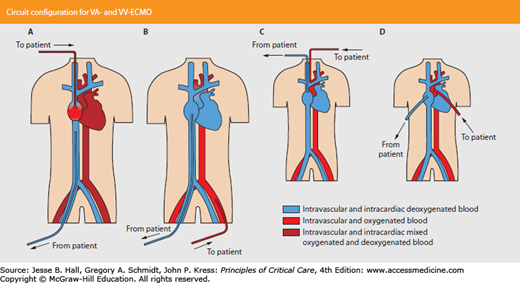
Veno-arterial ECMO (VA-ECMO), which provides both respiratory and cardiac hemodynamic support, is uncommonly required for respiratory failure and severe hypoxemia. But in patients with severe refractory shock requiring high-dose vasopressors (such as in severe septic shock), VA-ECMO may be advantageous. Blood is withdrawn from the venous circulation, oxygenated, and returned to the arterial circulation, bypassing the heart and lungs. For adults, accessing the femoral artery and vein is preferable, and percutaneous cannulation is usually feasible (Fig. 53-6). Perfusion to the ipsilateral leg will be impaired, and a reperfusion cannula to ensure adequate distal circulation to the lower extremity may be required.42
FIGURE 53-6
ECMO circuit configuration for VA-ECMO versus VV-ECMO. A. VV-ECMO; B. VA-ECMO, femoral cannulation; C. VA-ECMO, carotid cannulation; D. VA-ECMO, thoracic cannulation. (Reproduced with permission from Gaffney AM, Wildhirst SM, Griffin MJ, Annich GM, Randomski MW. Extracorporeal life support. BMJ. November 2, 2010;341:c5317.)
An initial bolus of heparin (100 units/kg) is administered before ECMO cannula insertion. Systemic anticoagulation with unfractionated heparin is commonly required during ECMO to avoid thrombus formation in the circuit. Anticoagulation is titrated by measurement of whole blood activated clotting time (ACT) and/or serial partial thromboplastin time (PTT). Our current protocol is the use of a continuous heparin infusion to target an activated PTT of 40 to 50 seconds. However, if the patient is at high risk for bleeding, or has had a bleeding complication, anticoagulation is held and reevaluated every 4 hours. We have had a number of patients who have had heparin held for days without circuit thrombosis. There is significant variability in protocols for anticoagulation across ECMO centers. Patients who have or develop heparin-induced thrombocytopenia can be managed with direct thrombin inhibitors for anticoagulation for ECMO.
Oxygenation and oxygen delivery are now primarily related to the ECMO blood flow rate through the oxygenator and this is titrated to achieve SaO2 >85% and SvO2 >65%. Ventilation is managed by titrating the sweep gas flow to remove carbon dioxide, and this is done slowly to avoid rapid arterial pH changes. There is significant center variability in hemoglobin and platelet targets on ECMO. Traditionally, ECMO was conducted with a high hemoglobin target to ensure adequate oxygen delivery in the face of relative hypoxemia, and in the CESAR Trial a hemoglobin target of >14 g/dL was the protocol. Our current adult VV-ECMO protocol targets include a hemoglobin of 10 g/dL or greater (increased to 14 g/dL, when unable to achieve adequate flow or reduced SvO2). Our platelet count target is >100,000 µL (same as CESAR trial) and is increased if bleeding complications occur.
ECMO allows for a decreasing of mechanical ventilator settings to nondamaging “rest” levels. Ventilator settings are decreased significantly, dependent on the adequacy of VV-ECMO support. Lung protection and reduction of VILI are the primary goals. Derecruitment occurs, and the chest radiograph “whites out.” Optimal lung-protective ventilatory strategies in these patients, as in severe ARDS patients due to other etiologies, focus on limiting end-inspiratory plateau pressure (Pplat) to <28 cm H2O and tidal volumes to <6 mL/kg of predicted body weight with provision of optimal positive end-expiratory pressures (PEEP) for alveolar recruitment. We have traditionally used a pressure control mode with peak inspiratory pressure of 20 to 25 cm H2O, PEEP 10 cm H2O, RR 10, and FiO2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree