Healthy individuals are usually unaware of their resting heartbeat, but persons suffering from somatization disorders (see
Chapter 230) may find the normally imperceptible heartbeat of daily life an unpleasant sensation. For most, awareness of heartbeat typically ensues when there is a sudden change in rate or rhythm, increase in stroke volume or contractility, or unusual cardiac movement within the thorax. Although acute changes in heart rate or rhythm may be noted, chronic dysrhythmias often go unnoticed. Disturbances of heartbeat may originate supraventricularly or ventricularly. Increased
automaticity and
reentry are among the basic mechanisms. The dysrhythmia itself may confer increased risk or represent a manifestation of underlying heart disease.
Supraventricular Dysrhythmias
As a group, arrhythmias originating in the atrium or junction tend to be less worrisome than those that originate in the ventricles, but atrial dysrhythmias may be manifestations of important underlying heart disease and sometimes can cause hemodynamic compromise or predispose to other complications (e.g., systemic embolization), making them important to identify and address.
Atrial Premature Beats
Premature atrial beats (APBs) are usually imperceptible, but awareness of a rhythm disturbance derives from the pause in rhythm, increased ventricular filling time, and the resultant forceful beat that follows the premature beat. A “turning over” or “flopping” of the heart may be reported. Premature atrial beats tend to be most noticeable when the heart rate is slow and the patient is lying in bed supine or in the left lateral decubitus position. If there is AV dissociation and atrial contraction occurs against a closed atrioventricular (AV) valve, then a “pounding in the neck” or a sudden bulging of the neck veins (jugular cannon venous A waves) may also be noted.
Atrial premature beats are a common occurrence of everyday life and often increase in frequency with the use of caffeine, nicotine, or alcohol. In most instances, they are harmless, but they can trigger atrial fibrillation and a rapid reentrant tachycardia in susceptible persons. They may represent increased atrial automaticity due to underlying heart disease (e.g., ischemia, cardiomyopathy, advancing valvular disease). A high frequency of premature atrial beats portends development of atrial fibrillation. Characteristic electrocardiographic (ECG) features include a premature and abnormally configured P wave followed by a narrow QRS complex and a resetting of the sinoatrial (SA) node before the next sinus-conducted beat (resulting in a “noncompensatory” pause in heart rhythm). If the APC occurs while the ventricles are still partially refractory, there may be aberrant conduction through the ventricle resulting in a widened QRS. Such APCs with aberrancy can mimic ventricular premature beats (VPBs) but are usually differentiated from the latter by a preceding P wave and the absence of a full compensatory pause.
Sinus Tachycardia
Excess adrenergic stimulation results in increased contractility and sinus tachycardia, which may present as palpitations with a fast regular rhythm. Onset can be abrupt; resolution is usually more gradual. A constant rapid pounding at rest is felt by patients with
hyperkinetic states (e.g., fever, severe anemia, hyperthyroidism, anxiety, agitated depression) due to the catecholamine-induced increase in contractility and stroke volume. Patients with anxiety-induced palpitations often have an underlying
panic disorder (see
Chapter 226); typically, they have difficulty telling whether the palpitations or the anxiety came first. Unappreciated is the high frequency of other supraventricular dysrhythmias in this group of patients (see later discussion). An uncommon variant of sinus tachycardia,
inappropriate sinus tachycardia, is believed to represent a hypersensitivity to catecholamine stimulation.
Hyperthyroidism may have a palpitation presentation similar to that of anxiety (see
Chapter 103); it may also cause atrial fibrillation (AF) (see later discussion). In rare instances, the source of adrenergic outpouring is a
pheochromocytoma. Its incidence is less than 0.1%, with about half of cases presenting as paroxysms of palpitations, hypertension, perspiration, tremor, nervousness, and other signs of adrenergic stimulation. Episodes are often spontaneous in origin but may be triggered by emotion and thus mimic an anxiety attack. An
insulin reaction can produce a similar clinical picture (see
Chapter 102), driven by an outpouring of catecholamines. A regular rhythm without tachycardia is noted in cases of valvular disease accompanied by large stroke volumes, as in
aortic regurgitation.
Other Regular Supraventricular Tachycardias
Attacks of palpitations that are regular in rhythm and rapid in rate may also be due to paroxysmal supraventricular tachycardia (SVT), sometimes referred to as paroxysmal atrial tachycardia. There are two basic mechanisms. In atrioventricular nodal tachycardia, which is the most common SVT, there are two functionally distinct conduction pathways in the nodal area, which enable a reentrant circuit to develop, producing ventricular response rates as high as 160 to 180 beats/min. If the atria in this rhythm disturbance are unaffected by the nodal rhythm disturbance, there can be AV nodal dissociation manifested as rapid regular pounding in the neck. The condition is considerably more common in women than men and occurs in a wide variety of patients, including those with normal hearts, sick sinus syndrome, mitral valve prolapse and other forms of valvular disease, coronary artery disease, and cardiomyopathy. Onset may occur in the setting of emotional stress (e.g., panic attack). Some patients note that standing up after bending over may bring on an episode that can be terminated by lying down.
In the second form of SVT with a regular rhythm, AV reciprocating tachycardia, there is a large macroreentrant circuit, which involves the atrium, the AV node, an accessory pathway (e.g., the bundle of Kent or James), and the ventricle. The preexcitation syndromes (so called because of their short ECG PR intervals), Wolff-Parkinson-White (WPW) and Lown-Ganong-Levine syndromes, operate by the reciprocating mechanism. An ECG hallmark of WPW is the delta wave at the beginning of the QRS, indicative of aberrant conduction through the accessory bundle; the QRS may be widened considerably during a run of SVT and mimic a ventricular dysrhythmia, especially when WPW is complicated by a very rapid ventricular response rate, as in atrial flutter or AF (see later discussion). Any SVT with a very rapid ventricular response rate may seriously compromise cardiac output in a patient with underlying heart disease and cause chest pain, dyspnea, profound weakness, or even loss of consciousness.
The onset of SVT is characteristically sudden and may be precipitated by excess alcohol, emotional upset, or strenuous exertion. Caffeinated beverages are less of a risk factor. SVTs may be initiated by the occurrence of premature beats that alter conduction in the normal pathway. Paroxysms cease when the conducting properties of the reentrant circuit are disturbed by changes in vagal tone, hence the report by patients of ability to terminate attacks by undertaking the Valsalva maneuver. Resolution is typically abrupt. Syncope is uncommon but may occur at the outset if the rate is very rapid and/or there is acute vasodilation.
Some of the conditions associated with SVT are responsible for other dysrhythmias as well. For example, almost half of patients with sick sinus syndrome experience heart block or marked bradycardia in addition to bouts of SVT.
Paroxysmal Atrial Fibrillation and Other Irregular Supraventricular Tachycardias
The sudden onset of palpitations with an irregularly irregular rhythm and rapid rate typifies paroxysmal AF, which occurs in a host of settings, including acute alcohol excess (“holiday heart”), infection, hyperthyroidism, sick sinus syndrome, WPW syndrome, cardiomyopathy, and acute worsening of ischemia or congestive heart failure (CHF); the condition is also found among otherwise healthy young people (“lone” atrial fibrillation [LAF]). High levels of circulating catecholamines may trigger AF, especially in someone with underlying organic heart disease. A common precipitant is exercise or the termination of exercise with its surge of vagal tone. Chronic AF usually does not produce palpitations. Runs of atrial flutter with a more regular rhythm may be interspersed with PAF and mimic AF when AV block is variable. This unstable arrhythmia often shares a common valvular, ischemic, or cardiomyopathic pathophysiology with AF and frequently reverts to it.
An irregularly irregular tachycardia may also be seen if there are runs of multifocal atrial tachycardia (MAT), which takes place in the context of severe pulmonary disease, particularly when there is an acute fall in oxygen tension or pH. Frequent atrial or ventricular premature contractions can lead to a similarly irregular rhythm and rapid rate.
Ventricular Dysrhythmias
Ventricular dysrhythmias often occur in the context of worrisome underlying heart disease and may be a harbinger of hemodynamic collapse and sudden death. However, not all ventricular rhythm disturbances are associated with increased cardiac morbidity and mortality.
Ventricular Premature Beats
Like most premature beats, those originating in the ventricles are often felt as a consequence of the forceful beat that follows the pause and increased ventricular filling after the premature
beat (see prior discussion). Cannon “a” waves may also ensue and be felt as an impulse in the neck. Electrocardiographically, VPBs are characterized by a widened QRS, no preceding P wave, and the presence of a full
compensatory pause (there is usually no resetting of the SA node, so the next ventricular depolarization must await the next regular discharge from the SA node). ECG differentiation of VPBs from APCs with aberrancy can be challenging because both cause a widened QRS, but the absence of a preceding P wave, the presence of a full compensatory pause, and a QRS duration greater than 140 msec are helpful. VPBs of right ventricular origin have a left-bundle pattern to the QRS; those arising from the left have a right-bundle pattern.
In the absence of overt underlying heart disease, most VPBs (even when frequent or complex) have little prognostic significance. Even when they occur during exercise stress testing, VPBs do not appear independently to confer a significant increase in cardiac risk, although the arrhythmia may be a sign of underlying cardiovascular disease. However, frequent VPBs that occur principally during the recovery phase of stress testing do confer an independent and significant increase in long-term mortality risk (the hazard ratio approaches 1.5). Such recovery-phase ventricular irritability (which is believed to be related to attenuated vagal reactivation) is also associated with reduced ejection fraction.
Ventricular Tachycardia
Ventricular tachycardia (VT) is among the most worrisome of dysrhythmias related to palpitations and is associated in some instances with the risk of sudden death. Nonetheless, not all VT represents life-threatening disease. Nonsustained ventricular tachycardia (NSVT) may occur in otherwise normal persons (idiopathic ventricular tachycardia), as well as in those with underlying heart disease. In truly normal subjects, NSVT is not associated with an increased risk of sudden death; however, any NSVT that compromises cardiac output may lead to dizziness, near-syncope, or loss of consciousness. Potentially serious VT may occur in the context of clinically evident heart disease, as well as in patients with important hereditary defects but little evidence of structural pathology.
VT in the Setting of Overt Heart Disease.
Most worrisome are runs of nonsustained or sustained VT occurring in the context of overt underlying heart disease. Such patients have an increased risk of sudden death, especially those with ischemic heart disease, hypertensive heart disease with left ventricular (LV) remodeling, dilated and hypertrophic cardiomyopathies, and hemodynamically significant valvular disease. The clinical picture is usually dominated by manifestations of the underlying heart disease but may include palpitations, near-syncope, and syncope. Such patients have a lowered threshold for VT and are especially susceptible to hypokalemia, hypomagnesemia, and medications that predispose to dysrhythmias (e.g., digitalis, methylxanthines, tricyclics).
VT in the Setting of a Normal-Appearing Heart.
More difficult to recognize are patients with VT who manifest little evidence of underlying heart disease but still have an increased risk of an adverse cardiac event. Some have otherwise clinically silent heart disease (e.g., ischemia, cardiomyopathy); others harbor genetic mutations or acquire a predisposition to serious ventricular dysrhythmias by virtue of medication use or metabolic disruption. Clues to this underlying pathophysiology include the use of drugs that impair repolarization, a family history of early sudden death, recurrent syncopal episodes, and exerciseinduced VT. Included in this group are persons with prolonged QT interval, right ventricular outflow tract VT, right ventricular cardiomyopathy, and Brugada’s syndrome.
Prolonged QT Interval. Prolongation of the QT interval (QTc >460 msec in women and >440 msec in men) is increasingly recognized as an important cause of VT, particularly the potentially lethal polymorphic VT referred to as torsade de pointes. Prolongation of the QT interval represents a disorder of myocardial repolarization, which may be acquired or hereditary.
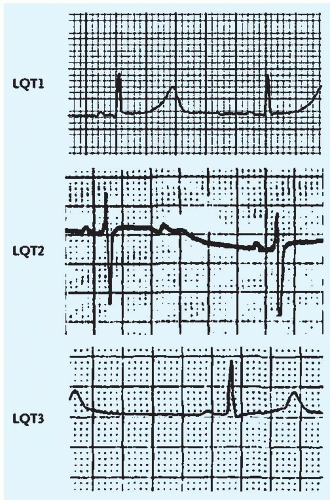
In
hereditary forms, referred to as
long QT syndrome, mutations in sodium and potassium channel genes (designated LQT1 to 6) are associated with a spectrum of repolarization abnormalities and susceptibility to VT, torsade, and ventricular fibrillation (VF) (
Fig. 25-1). Patients are typically young (under the age of 40 years) and may have a history of syncope with or without premonitory palpitations and a family history of sudden death or recurrent syncope, often without warning. Risks of VT and cardiac death vary greatly among persons with hereditary forms of the condition; the overall risk is about 5%. The risk varies with the site of mutation, degree of QT prolongation, and gender. In patients with a history of syncope, 10-year mortality rates approach 50%. Precipitants include severe emotional stress and very vigorous exercise.
In
acquired forms, precipitants to QT prolongation include bradycardia, older age, LV dysfunction, ischemia, hypertrophy, medications, hypokalemia, hypomagnesemia, hypothyroidism, liquid protein diets, and eating disorders that cause starvation. The ECG criteria for QT prolongation are a corrected QT
interval of greater than 450 msec in men and of greater than 460 msec in women. Other ECG features include T-wave alternans, notched T waves, and prominent U waves. Among the medications implicated include the antihistamines
terfenadine and
astemizole (especially when taken in excess or concurrently with agents that impair mitochondrial cytochrome P450 activity), as well as
class IA and
class III antiarrhythmics, macrolide antibiotics, antipsychotics, antifungal agents, and
tricyclic antidepressants. Persons with a hereditary predisposition to QT prolongation are believed to be especially susceptible to drug-induced disease.
Right Ventricular Outflow Tract VT.
Right ventricular outflow tract VT occurs in persons without evidence of structural heart disease. The VT originates from the septal portion of the right ventricular outflow tract. Patients may report exercise-induced palpitations, dyspnea, or syncope. The right ventricular origin of the VT is manifested by its left-bundle pattern on ECG.
The Brugada Syndrome.
The Brugada syndrome is a hereditary, autosomal dominant syndrome due to a mutation in a gene important to the functioning of the heart’s sodium channels. Male patients outnumber female patients by 9:1. Affected persons manifest no overt heart disease, but they may have a family history of sudden death, and their ECG may show characteristic features of right-bundle-branch block and ST-segment elevation in leads V1 to V3. Affected persons have a high rate of sudden death, especially during sleep. Mortality is high (up to 10%/year).
Right Ventricular Cardiomyopathy (Arrhythmogenic Right Ventricular Cardiomyopathy).
Right ventricular cardiomyopathy is a subtle cardiomyopathic cause of VT with an increased risk of sudden death. Right ventricular muscle is replaced with fibrofatty tissue. Besides sudden death, affected persons, typically in their 20s to 30s, may experience syncope or report exercise- or non-exercise-induced palpitations due to VT. The ECG may show T-wave inversions in leads V1 to V3 and right-bundle-branch block (with or without ST-segment elevation), which is believed to be due to poor conduction through the involved fibrofatty myocardium of the right ventricle. On stress testing, there can be ventricular ectopy with a left-bundle pattern, indicative of a right ventricular origin of the VPBs. The echocardiogram may reveal a dilated, poorly contractile right ventricle. Magnetic resonance imaging scanning reveals fatty displacement of normal myocardial tissue.