Component
Visceral
Somatic
Nerve fiber
Ad, C
Ab [allodynia], Ad, C
End organ silent nociceptors
Yes (~80 %)
Yes (~20 %)
DH laminar synapse
I, V, X
I-to-V and X
Trigger/insult
Distension
Direct trauma
Ischemia
Ischemia
Character/location
Dull, poorly localized
Sharp, precisely localized,
Relation to stimulus
Referred site
Radiation along the nerve
Associated symptoms
Nausea, vomiting, sick feeling
Tenderness, soreness
Stimulus dependent summation
Intensity dependent summation
No
Sensitization
Mechanical
No
1st Hyperalgesia
Yes
Yes
2nd Hyperalgesia
Yes [at referred site]
Yes
Sensitization
Mechanical-common
Thermal-common
Mechanical-uncommon
In summary, characteristics of visceral pain include:
Referred to body wall
Diffuse & poorly localized
Accompanied by increased motor & autonomic reflexes
Severity of pain doesn’t always reflect disease severity (e.g., mild/no pain in colon cancer, severe pain when passing a stool in IBS)
Not evoked from all organs (solid < hollow)
Not always linked to injury [functional disorders]
Neurophysiology and Neuroanatomy of Abdominal Pain
Most of the knowledge on chronic pain has derived from research on somatic nociceptors. As a result, the unique visceral sensory components are typically less understood which in turn results in less efficacy of the treatment of visceral pain in comparison to that of somatic pain.
Visceral Neurophysiology
Visceral afferent nerves differ functionally from non-visceral afferents. Visceral nociceptors (Table 4.1) are:
Mechanically insensitive
Not sensitive to physiological/supraphysiological stimuli
Recruited only in pathological situations
Sensitive to inflammatory mediators
Activated by distension, pressure, and ischemia
The principal visceral afferents are anatomically associated with sympathetic and parasympathetic fibers [splanchnic afferents, pelvic nerve afferents]. There are two types of peripheral afferent nerve endings: (a) low threshold; and (b) the largest population—high threshold [reserve, inactive, nonfunctional usually]. Low threshold peripheral organ mechanoreceptors [also called “wide dynamic range”] encode organ distension from low physiologic pressures to high noxious pressures. They are insensitive to touch, cut and generally any form of mechanosensation. The high threshold endings become active upon development of end organ pathological processes such as local inflammation and stimulation by chemokines and interleukins. This capacity for neuroplasticity is the basis of peripheral end organ sensitization, which results in central sensitization and expansion of receptive fields. Furthermore, the ability to localize the source of pain (spatial resolution) in visceral pain is poor due to two factors: (1) Viscera are relatively sparsely innervated in comparison to somatic structures (nearly 1:10 ration in comparison to somatic structures); and (2) Secondary spinal neurons receive convergent input from both viscera and skin. Pain pathways involved in visceral nociception are maintained by both efferent mechanisms [sympathetically maintained] and afferent visceral sensory mechanisms. A study by AK Houghton and colleagues concluded that dorsal column pathways play an important role in the processing and relaying of pancreatic visceral nociceptive information [1].
Morphological Consideration
Neuronal cell types are largely defined by size. Large “light” neurons have A fibers and small “dark” neurons have C-fibers. Fiber size is directly related to the degree of fiber myelination, which is, in turn, directly related to conduction velocity. Thus the large, myelinated A fibers have the fastest conduction velocity whereas the small, unmyelinated C fibers have the slowest conduction velocity [2, 3]. Comparing nociceptive to non-nociceptive afferent neurons [4], nociceptive neurons were found to have longer action potential duration and slower maximum firing rate. These properties appeared to be graded according to the conduction velocity with the slowest (C) fibers having the longest action potential and the least rate of fiber firing (C > A-δ > A-β/α). Up to 80 % of visceral dorsal root ganglia cell somata are C-fibers, and the rest Aδ fibers. Aβ fibers are rarely encountered on viscera. In comparison, only 7–17 % of C-fibers are found at the L4 DRG part of the sciatic nerve. Spinal visceral afferents terminate in the superficial dorsal horn, lamina V and around the central canal (lamina X). Somatic cutaneous afferent neurons terminate in the superficial lamina of the dorsal horn and lamina V which explains the convergence of visceral and somatic stimuli at the level of second order neurons.
Neuroanatomy of Abdominal Visceral Sensory System
As discussed earlier, abdominal viscera receive dual innervation from the afferent and efferent system.
Sympathetic Nerves
Preganglionic sympathetic fibers, which control the function of the abdominal viscera, have their cell bodies in the spinal cord segments T5-to-L2. Their axons pass via the white rami communicates and through the sympathetic trunk without synapsing to become the splanchnic nerves. The splanchnic nerves end in the prevertebral ganglia where they synapse with postganglionic neurons. The postganglionic neurons leave the celiac and related ganglia, and together with the sensory and vagal fibers, reach the end organs. Each organ is supplied by sympathetic nerves, which originate in specific spinal cord segments.
Parasympathetic Nerves
The upper abdominal viscera are supplied by parasympathetic fibers from the cell bodies in the dorsal motor nucleus of the vagus, which pass via the prevertebral visceral plexuses to end in the terminal ganglia in the walls of the viscera. Parasympathetic fibers do not transmit pain sensations.
Sensory Nerves
Sensory nerves supplying the viscera accompany both the sympathetic and parasympathetic fibers. Sensory fibers, which are components of the splanchnic nerves, enter the spinal cord via the posterior roots of the T5 to L2 nerves. They serve as the primary pain pathways from the abdominal viscera.
Diagnostic and Prognostic Nerve Blocks in Chronic Abdominal Pain
According to the International Association for the Study of Pain (IASP) taxonomy, CAP can be classified into:
I.
Abdominal wall pain
II.
Abdominal pain of visceral origin
III.
Abdominal pain syndromes of generalized diseases
IV.
Pain of psychological origin
V.
Chronic pelvic pain syndromes
VI.
Diseases of the bladder, uterus, ovaries, testes, and prostate, and their adnexa
VII.
Pain Perceived in the rectum, perineum, and external genitalia of nociceptive or neuropathic cause
Given the difficulty introduced by neurophysiological, neuroanatomical, and behavioral factors, and in order to increase the diagnostic accuracy as well as improve the prognosis of any subsequent invasive treatments, a pain physician may employ nerve blocks (Fig. 4.1.). Many techniques derived from regional anesthesia have consistently stood the test of time and preserved their clinical utility as diagnostic tools. However, these techniques are not to be used as sole approaches to arrive at clinical diagnoses or clinical decision-making. Rather, the information obtained from nerve blocks should be used in conjunction with the patient’s history, physical examination and labs, imaging and GI evaluation to guide future management interventions (Fig. 4.2.). On their own and without taking into account the whole clinical picture, nerve blocks may be of limited value in chronic abdominal pain. In addition, choosing the appropriate nerve block in the proper patient is critical for the usefulness of these interventions.
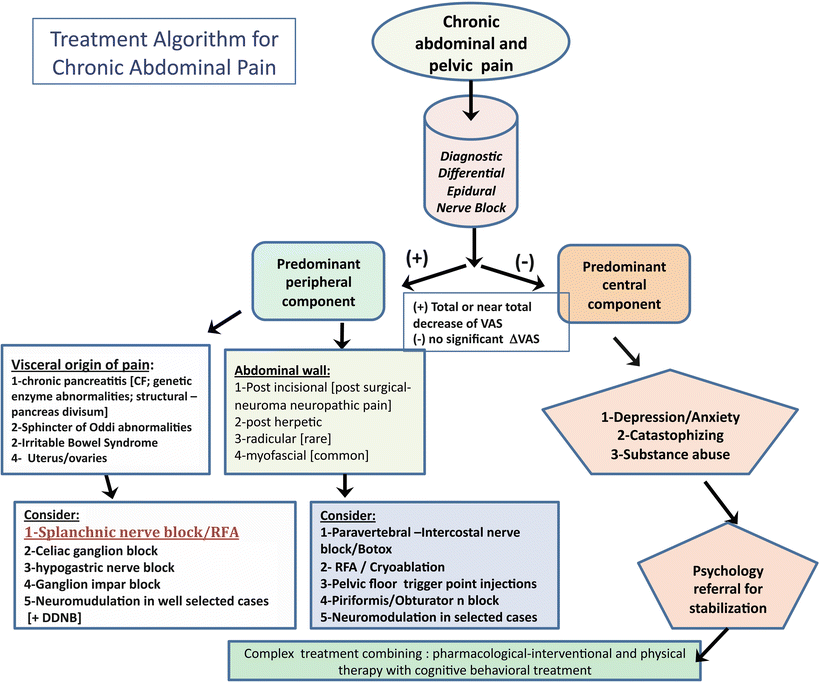
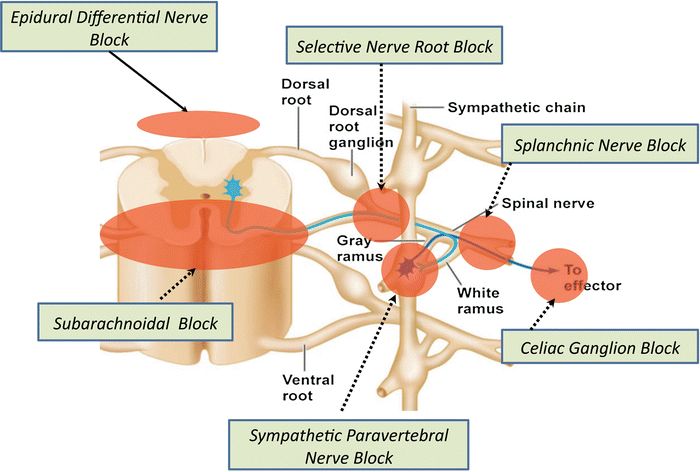

Fig. 4.1
Common diagnostic nerve blocks. Neuraxial and peripheral nerve block sites of action. Neuroanatomical and neurophysiological specificity is hypothesized to make diagnostic blocks valuable tools in aiding pain physicians in the diagnosis and treatment of visceral abdominal pain patients

Fig. 4.2
Descriptive algorithm for diagnosis and treatment of chronic abdominal pain
In general, nerve blocks may be of therapeutic, diagnostic, or prognostic value. Often, initial nerve block interventions in CAP are done for diagnostic or prognostic purposes. These can utilize an anatomic or pharmacologic approach to delineate likely processes involved in CAP maintenance. Two types of nerve blocks have a significant role in the differential diagnosis and prognosis of CAP:
(a)
Neuraxial diagnostic blocks;
(b)
Peripheral nerve blocks: splanchnic nerve block (SNB), celiac nerve block, superior hypogastric nerve block, ganglion impar blocks, paravertebral nerve blocks, and intercostal nerve blocks.
Transversus Abdominis Plane (TAP) blocks and blocks of the ilioinguinal and genitofemoral nerves may be performed for diagnostic or therapeutic reasons in postsurgical pain or abdominal nerve entrapment situations. They are discussed elsewhere.
Neuraxial Diagnostic Differential Neural Blockade
Nearly 80 years ago Gaser & Erlanger demonstrated that less cocaine is needed to stop conduction in thin myelinated A nerve fibers than in thicker ones. Since then, decades of research have documented differential local anesthetic susceptibility of A-, C-, and B fibers. There are two approaches to achieving this differential block of nerve fibers: an anatomic approach and a pharmacologic approach. The anatomic approach is based upon the notion that there is enough anatomic separation of the nerve fibers (afferent and efferent) to allow selective blockade of particular painful abdominal structures. The pharmacologic approach takes advantage of the fact that sympathetic and somatic nerve fibers exhibit different sensitivities to local anesthetics, thus the injection of various concentrations of local anesthetics would allow for a differential blockade of the fibers [5]. By observing the analgesic and anesthetic responses to injections of normal saline (placebo) and different amounts of local anesthetics, pain may be distinguished as visceral or non-visceral in origin.
Diagnostic Differential Nerve Block
While differential neural blockade has been observed using both subarachnoid and epidural anesthesia, the epidural technique was found to be more advantageous and controllable in the differential diagnosis of CAP. Epidural diagnostic differential nerve block (DDNB) is the most useful tool in the armamentarium of the Pain Medicine physician for diagnostic purposes in CAP.
Purpose: The goal of DDNB is to delineate underlying pain mechanisms involved in maintenance of the chronic pain state [6]. Epidural differential nerve blockade is performed with injections of placebo, and differing amounts of local anesthetics through an epidural catheter to achieve surgical anesthesia in the afferent neuronal distribution that overlaps the patient’s site of pain
The Role of Local Anesthetics
The duration of nerve conduction block is dependent upon the protein binding affinity of the local anesthetic while lipid solubility determines potency of the agent. Thus, lipophilic amide-based local anesthetics with high protein binding properties, such as bupivacaine, may produce longer neuronal blockade and at lower concentrations than their less lipophilic ester-based counterparts such as 2-chloroprocaine. An amide local anesthetic with lower lipophilicity and less protein binding affinity than bupivacaine, such as lidocaine, may also be useful. Because of these reasons, 2 % lidocaine or 2-chloroprocaine are often used for epidural diagnostic differential neural blockade. In vivo studies have shown that a differential susceptibility exists based upon fiber size regardless of the type of local anesthetic used [7]. A-α fibers have been shown to be less sensitive to local anesthetics, regardless of type, in comparison with the smaller C or A-δ fibers. The order of susceptibility to local anesthetic blockade from most to least susceptible is: B < C < Aδ < Aγ < Aβ < Aα [5, 8–10].
Differential Neural Blockade: Epidural Approach
The classic spinal approach involved placing the patient in the lateral decubitus position and administering saline and various concentrations of local anesthetics through a spinal needle. Given drawbacks to this conventional subarachnoid differential technique, the epidural approach was suggested by Raj in 1977 [5] in an attempt to avoid positional issues and the possibility of a post-dural puncture headache following differential spinal (intrathecal) blockade [11]. Raj’s technique was identical to that of the subarachnoid classic approach in that a placebo solution and increasing concentrations of local anesthetic were injected into the epidural space and the patient was observed for the onset of pain relief with the onset of neural blockade of the various fibers. However, because the injections occurred within the epidural space as opposed to the subarachnoid space, the local anesthetic utilized was lidocaine in concentrations considered be the mean concentrations leading to blockade of the various neural fibers within the epidural space.
Raj’s epidural technique brought about two problems: (1) the onset of neural blockade is slower in the epidural space, thus Raj’s technique is more time-consuming than the classic subarachnoid approach; and (2) local anesthetic injected into the epidural space gives even less discreet endpoints than injection into the spinal space, leading to possible misinterpretation of results. The two disadvantages of Raj’s technique can be circumvented in the same way the modified spinal approach circumvented the same drawbacks to the classic spinal approach as described below.
Modified Epidural Procedure
The patient is placed in the supine, lateral, or sitting position and the back is prepped and draped in the usual sterile fashion. An epidural needle is utilized to gain access to the epidural space utilizing a loss of resistance technique. At this time, an epidural catheter is placed for the administration of the inactive and active solutions. Preservative free normal saline is injected initially as a placebo and is followed 10–15 min later by the injection of 2 % or 3 % chloroprocaine (or 2 % lidocaine) in incremental doses until a T4 sensory level is achieved. The needle/and catheter are removed. Prior to injecting the solutions as well as after each injection, the same observations mentioned in Table 4.2 are assessed.
Table 4.2
Transient Differential: Time Sequence of functional impairment at onset of Differential epidural block and expected change in VAS score
Effect observed | Saline solution | Chloroprocaine 3 % |
|---|---|---|
Vasomotor | [–] | +/−sympathetolytic |
Warmth and pinprick | [–] | |
Cold | [–] | |
Touch/Motor | [–] | |
Change in pain intensity [DVAS] | [–] | [+] or [−] |
Interpretation
If the patient experiences pain relief after injection of saline, the pain relief is considered to be influenced by suggestion and reflects a place. If the patient does not experience pain relief following the injection of the anesthetic with a resulting appropriate sensory level, the pain is considered to be central in origin. However, when pain relief does occur following injection of the local anesthetic, the pain is considered to be organic in nature. Whether the pain is sympathetic or somatic in origin cannot be delineated until neural function begins to return. If the pain reappears with the return of the perception to pinprick, the pain is deemed to be somatic. If the return of pain occurs well after the return of sensation the pain is consider sympathetically mediated or visceral in origin.
Limitations of Epidural DDNB
Does not measure precisely the extent of nerve fiber blocked.
More than one fiber type is simultaneously blocked leading to misinterpretation of the results.
It is impossible to blind the patient, as such a placebo effect is significant especially if it occurs on repeat differential block procedure.
Neuroplasticity changes could lead to block of nerve conduction at subanesthetic concentrations altering result interpretation.
Significant behavioral components of chronic abdominal pain patients could influence patient response.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






