Trauma
Craniofacial trauma
Nasal/nasoseptal fracture
Digital trauma (nose picking)
Foreign body
Nasal instrumentation (Nasogastric tubes, nasal intubation)
Barotrauma
Postsurgical trauma
Structural
Nasal septal deviation
Septal spur
Septal perforation
Necrosis of septum—intranasal illicit drugs
Neoplasms (local or systemic)
Juvenile angiofibroma
Nasal polyposis
Inverted papilloma
Hemangio (glomangio)-pericytoma
Malignant neoplasm of the nasal cavity or paranasal sinus
Infections, inflammatory or autoimmune
Rhinosinusitis
Nasal diphtheria
Wegner’s granulomatosis
Disorders of coagulation
Acquired
Antiplatelet therapy
Anticoagulation
Thrombolytics
Cirrhosis (alcohol or infectious)
Uremia
Systemic
Leukemia
Hemophilia
Von Willebrand or other inherited clotting disorder
Sickle cell disease
Vascular
Hypertension
Hereditary hemorrhagic telangiectasia
Preoperative Preparation
Traditionally nasal hemorrhage has been divided into anterior and poster epistaxis based on the site of the bleeding. The nasal septum and lateral nasal wall have a rich vascular supply based on an anastomosing network of the internal and external carotid circulations. The external carotid artery blood supply to the nasal septum is derived from the septal branches of the superior labial division of the facial artery and the greater palatine artery; posteriorly the septum is supplied by the dorsal septal branches of the sphenopalatine artery. Superiorly the nasal septum is supplied by the anterior and posterior ethmoidal branches of the ophthalmic division of the internal carotid artery. The most frequent site of epistaxis is the dense vascular plexus overlying the anterior cartilaginous septum or Kesselbach’s plexus, formed by the anterior ethmoidal and anterior septal arteries. The blood supply to the lateral nasal wall is derived from terminal divisions of the sphenopalatine artery with smaller contributions anteriorly from the anterior ethmoid and superiorly from the posterior ethmoid arteries. The most common site of posterior epistaxis is the posterior lateral nasal wall at the terminal divisions of sphenopalatine artery at the base of the middle turbinate.
The treatment of the patient with epistaxis can be significantly streamlined and frustration for the managing providers reduced by assembling all the proper equipment prior to manipulating the nasal cavity. The patient needs to be examined in the sitting position. Examining patients supine will direct blood posteriorly into the pharynx and larynx precipitating coughing and making epistaxis control significantly more challenging. Necessary equipment includes a headlight, nasal speculum, suction, personal protective equipment, topical vasoconstrictors, absorbable hemostatic material, and nonabsorbable packing or tamponade devices (Table 16.2).
Table 16.2
Equipment for epistaxis management
Personal protective equipment |
Gowns, gloves, mask, protective eye wear |
Suction |
Yankauer or tonsil and Frazier (nasal) suction tips |
Headlight |
Kidney basin |
Ice water |
Equipment |
Nasal speculum, bayonet forceps |
Topical anesthetics and vasoconstrictors |
4 % plain lidocaine |
Epinephrine 1:10,00 topical |
Oxymetazoline |
Cotton balls or neurosurgical 1 × 3 neurosurgical pledgets |
Topical hemostatic agents |
Silver nitrate, Gelfoam sheet, Surgical sheet |
Vaseline impregnated gauze 0.5 × 72 in. |
Nonabsorbable tamponade sponges |
Anterior–posterior balloon packing device |
Foley catheter 20 cc balloon |
Management Strategy
Control of nasal hemorrhage should proceed in a systematic fashion from these least to most invasive methods (Table 16.3). The provider should assure durable hemostasis and not discharge the patient until after a minimum of 2 h of observation and no further bleeding is identified. Persistent poor control of anterior bleeding, need for multiple packing, bleeding through packing, or bleeding posteriorly into the pharynx indicate inadequate hemostasis and are indications for anterior–posterior packing, vascular intervention, and likely transfer.
Table 16.3
Treatment of epistaxis (escalation of invasive techniques)
Level 1 (site-directed therapy) |
Digital pressure and topic oxymetazoline |
Topical hemostatic agents |
Oxidized cellulose |
Microfibrillar collagen |
compressed gelatin |
Electrocautery |
Level 2 |
Nonabsorbable nasal packing |
Polyvinyl acetal (PVA) sponge |
Petrolatum gauze |
Balloon packing |
Level 3 |
Vascular control |
Endoscopic sphenopalatine and/or anterior ethmoid artery ligation |
Trans-antral sphenopalatine artery ligation |
Ligation of anterior ethmoid artery (medial canthotomy) |
External carotid artery ligation (extremely rare) |
Trans-catheter angiography and embolization |
When the rural surgeon is called to assist in managing an individual with epistaxis it is likely that the patient will have failed previous attempts at hemostasis and the hemorrhage will have become severe. Initial assessment and management should include the basic life support algorithm of establishing a secure airway, maintaining unobstructed spontaneous breathing, and confirming appropriate intravascular volume to maintain adequate circulation. The past medical history should be obtained with attention to a history of illicit drug use, systemic anticoagulation or antiplatelet therapy, bleeding diathesis, current medications, and ischemic heart disease. The patients should be examined sitting upright and leaning forward to direct blood anteriorly away from the pharynx with digital pressure pinching the naris alternating sides. This maneuver will help elucidate the side of the bleeding and whether the bleeding is anterior or posterior. The severity of the hemorrhage should be approximated by reviewing the history of duration of bleeding, estimated blood loss present in suction canisters, towels etc., and clinical indications of hypoxemia such as hypotension, tachycardia, and reduced oxygen saturation. Intravenous access, fluid resuscitation, supplemental oxygen or rarely transfusion of blood or blood products may be acutely necessary. Based on the severity of the bleeding, the patient’s comorbidities and hemodynamic status blood should be drawn for baseline hemoglobin and hematocrit levels, serum biochemistry studies, and type and cross match in the event of continued bleeding requiring transfusion of packed red blood cells, or platelets. Prothrombin time (PT), partial thromboplastin time (PTT), the internal normalized ratio (INR), and thromboelastogram (TEG) can also be obtained based on a history of the use of anticoagulation or antiplatelet medications or illicit substance abuse or an anticipated need to administer coagulation factors.
On rare occasions patients will present with uncontrolled severe epistaxis with blood literally pouring unabated from the nose and mouth. In this situation initial management is to place a Foley catheter into the nasopharynx, inflating the balloon with saline, placing gentle anterior traction on the catheter and seating the balloon in the nasopharynx by palpating the soft palate (Fig. 16.1). The surgeon can then proceed to bilateral anterior packing with vasoconstrictors as described below. Massive uncontrolled hemodynamically significant nasal bleeding, particular with oxygen desaturation or evidence of myocardial ischemia may require urgent orotracheal intubation to secure the airway and prevent continued aspiration of blood.
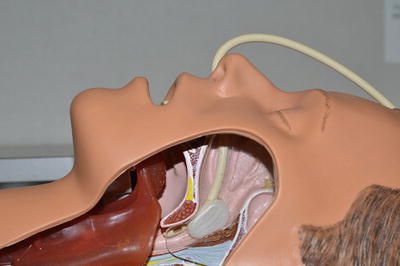

Fig. 16.1
Acute balloon tamponade of the posterior nasal cavity with Foley catheter
Once the initial resuscitation is complete the nasal cavity should be racked bilaterally with vasoconstrictor impregnated cotton balls or neurosurgical cotton pledgets. Packs are first placed along the nasal floor and layered superiorly. Effective topical vasoconstrictors include 0.5 % phenylephrine, epinephrine diluted 1:10,000, or 0.05 % oxymetazoline all of which can be mixed directly with 4 % plain (topical) lidocaine to achieve both intranasal hemostasis and anesthesia (Fig. 16.2). Historically, 4 % topical cocaine has been used to achieve intranasal hemostasis and anesthesia but it is more likely associated with hypertension, tachyarrhythmias, and agitation and has been replaced by these other agents.
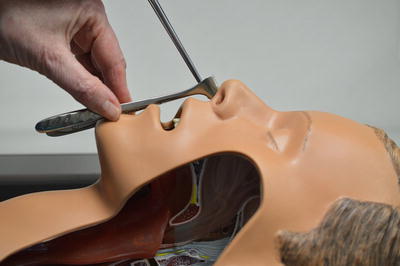

Fig. 16.2
Approach for instrumenting the anterior nose
With acute bleeding under control the lateral nasal wall and septum can be clearly inspected to identify potential bleeding sites. Headlight, nasal speculum, or an otoscope with the largest adult ear speculum, and suction will help clear blood from the nasal cavity and facilitate identification of the bleeding site(s). Optimum examination of the nasal cavity is performed with a 4 mm diameter 0° nasal endoscope.
Operative Techniques
Site-specific hemostasis can be performed with chemical or thermal cautery and supplemented with dissolvable hemostatic material. The nasal mucosal should be topically anesthetized as described above prior to any manipulation. Additional intranasal anesthesia can be accomplished anteriorly by injecting 1 % plain lidocaine into the lateral nasal wall anterior to the root of the middle turbinate. If more posterior anesthesia is needed a sphenopalatine nerve block is needed. This be performed by injecting 1 % plain lidocaine at the posterior aspect of the middle turbinate and directing it superiorly and laterally into the sphenopalatine foramen. If nasal bleeding precludes safe visualization of the lateral nasal wall, the sphenopalatine nerve can be anesthetized via an intraoral greater palatine nerve block. This is performed by first injecting lidocaine 1 % 1:100,000 with epinephrine into the mucosa of the hard palate 1 cm medial to the second molar tooth using a 25-gauge needle bent 60 % 2 cm from the tip. The needle is then advanced superiorly into the greater palatine canal and injecting 1.5–2 cc of local anesthetic resulting in anesthesia of the greater palatine and sphenopalatine nerves and subsequently the posterior lateral nasal wall.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








