CHAPTER 23 Epidural Steroid Injections
Description
Terminology and Subtypes
Epidural steroid injection (ESI) is a generic term used to indicate various types of injections that attempt to introduce one or more medications into the epidural space; not all ESIs involve corticosteroids. There are three main subtypes of ESIs that are named according to the method used to introduce therapeutic agents into the epidural space: (1) caudal ESI (CESI), (2) interlaminar ESI (ILESI), and (3) transforaminal ESI (TFESI) (Figure 23-1).1,2 ILESI is also termed translaminar ESI. TFESI is also termed selective nerve root injection or selective nerve root block. However, because the latter targets the spinal nerve root rather than the intervertebral disc, selective nerve root injections are typically performed to treat specific radicular symptoms rather than axial CLBP.
The term ESI is generic and can also apply to injections administered to the cervical spine. ESIs for chronic low back pain (CLBP) are referred to as lumbar or lumbosacral ESIs. The CESI and ILESI approaches are the most commonly used as they can generally be performed without fluoroscopy (also called image guided); there is a substantial likelihood of needle misplacement when administering CESI or ILESI without fluoroscopy (also called a blind approach).3,4 Whereas TFESIs often place medication around the posterolateral perimeter of the affected spinal level, CESIs and ILESIs are often unable to do so with accuracy. CESI and ILESI are consequently termed nonspecific ESI, whereas TFESI may be termed specific ESI.
History and Frequency of Use
Conventional medicine has commonly upheld the notion that 80% to 90% of low back pain (LBP) does not have any identifiable etiology and is thus termed nonspecific LBP. This belief has been predicated on the early work of Dillane and colleagues who could not detect an identifiable cause of LBP in 79% of males and 89% of females in a general clinical practice.5,6 Similar findings were presented by Nachemson, who could not identify a demonstrable pathoanatomic explanation for 85% of those with LBP.7 Valkenburg and Haanen reported finding objective evidence of disc prolapse in only 2% of patients with LBP, whereas 6% had objective findings of lumbago.8 Additional research conducted in the general asymptomatic adult population reported that lumbosacral spine abnormalities were routinely discovered by myelography, computed tomography (CT), and magnetic resonance imaging (MRI).9–11 The prevalence of lumbar degenerative findings generally increases with age.
It should be noted that Dillane and colleagues conducted their research before the advent of advanced diagnostic modalities. Studies using fluoroscopically guided, diagnostic spinal procedures have reported being able to differentiate the various sources of LBP. It has been reported that 30% to 50% of LBP is due to internal disc disruption syndrome, 13% to 19% can be attributed to sacroiliac (SI) joint dysfunction, and 15% to 17% is related to painful zygapophysial joints.12–15 If these findings are mutually exclusive, these results might suggest that 58% to 86% of LBP cases could potentially be attributed to specific diagnoses involving discs, SI joints, or facets. These findings also suggest that local injections of anesthetic, analgesic, or anti-inflammatory medication targeted at lumbosacral anatomic structures involved in the genesis of LBP may provide at least temporary symptomatic relief if successful.
The CESI approach was first described in 1901 by a French radiologist who injected diluted solutions of cocaine (commonly used as an anesthetic at the time) through the sacral hiatus to treat intractable LBP or sciatica.16 Half a century later, in 1957, Cappio investigated the therapeutic benefit of injecting corticosteroid into the epidural space using a caudal approach.17 The ILESI technique was first described by Pages in Spain in 1921, and the therapeutic benefits of this approach for LBP were investigated 40 years later.18–20 The first report of a lumbosacral TFESI appeared in the Italian literature when Robechhi and Capra reported using this approach to successfully treat lumbar and sciatic pain.21
ESIs are commonly used to manage CLBP in the United States.22 In fact, it has been reported that they are the single most commonly used injection procedure for CLBP by interventional pain specialists.23 TFESIs are widely used to manage symptoms of radiculopathy related to LBP.24 A study examining the prevalence of ESIs in Medicare patients reported an increase of 271% between 1994 and 2001, from 0.6% of the population to 2.1%.25 A related study reported substantial variations in the use of ESIs across various states, varying from 0.5% of Medicare enrollees in Hawaii to 4.0% in Alabama.26 Larger variations were noted between cities, ranging from 0.6% in Honolulu to 10.4% in Palm Springs.
Practitioner, Setting, and Availability
ESIs should only be performed by a physician trained in the safe and competent administration of these procedures, with advanced training in life support to address potential complications that could result from such injections. Weekend cadaver workshops may also be useful during medical residency before deciding to pursue additional fellowship training. CESI and ILESI have traditionally been taught with hands-on workshops. TFESIs are best learned during a rigorous, comprehensive 1-year interventional pain or spine fellowship. All ESIs should be performed in a setting with ready access to intravenous fluids, cardiac and pulse oximetry monitoring, and code carts, such as a private practice, ambulatory surgery center, or hospital-based surgery center. ILESIs and CESIs can be performed without fluoroscopic guidance and require fewer staff and support personnel. However, TFESIs require specialized equipment typically available only in specialized surgical centers or hospitals. Although ultrasonography has been used to guide injections in preclinical trials, further investigation is required to examine ultrasonography’s capacity to detect intravascular uptake of the injectate.27,28
Procedure
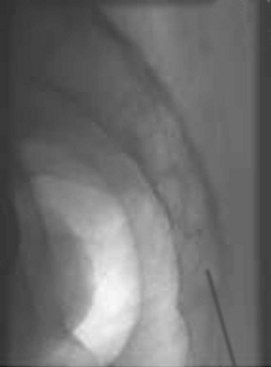
CESI is performed by placing a spinal needle through the skin and subcutaneous tissue, oriented almost vertically into the sacral hiatus (Figure 23-2). This technique can be performed with relative ease, by an experienced clinician, in relatively thin individuals. Because the sacral epidural space must be filled completely before the injected medication is distributed cephalad to eventually reach the lumbar epidural space, a relatively large volume of medication must be injected. Even when using a large volume, medication injected with CESIs rarely reaches the ventral epidural space or progresses cephalad beyond the L5-S1 segmental level.29
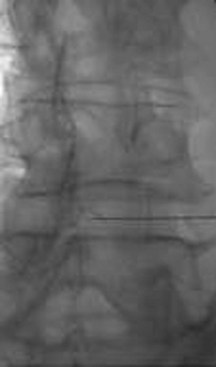
ILESI is performed by placing a spinal needle through the skin, subcutaneous tissue, and muscle, oriented inferior to superior and between adjacent spinous processes in order to reach the epidural space (Figure 23-3). This approach offers the potential advantage of delivering medication directly into the lumbar region rather than introducing it in the sacral region and hoping it will reach intended target cephalad. However, the ILESI approach is technically more demanding than the CESI. The medication is injected into the posterior epidural space without any assurances it will flow anteriorly to the ventral epidural space.30
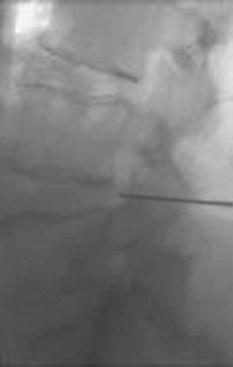
TFESI is typically performed under fluoroscopy by placing a spinal needle through the skin, subcutaneous tissue, and muscle, oriented from lateral to medial, in order to reach the lateral foramen of the targeted spinal nerve root (Figure 23-4). In contrast to CESI and ILESI, the medication injected with TFESI will reach the ventral epidural space in virtually every case.31 The instillation of therapeutic doses of corticosteroid into the anterior epidural space to maximally reach the targeted intervertebral disc is best accomplished by TFESIs rather than with ILESIs or CESIs.
Theory
Mechanism of Action
Evidence suggests that there is an increased production of proinflammatory mediators and cytokines because of disc herniation and higher levels of interleukin (IL)-6, IL-8, and prostaglandin E2 (PGE2) in degenerative, nonherniated painful discs.32–39 Cyclical mechanical loading of the disc coupled with inflammatory stimuli have increased PGE2 production by nuclear and annular disc cells in vitro, with a stronger reactivity in the latter models.40 Painful degenerative lumbar intervertebral discs have higher concentrations of sensory fibers—located in the end plate and nucleus—than nonpainful discs, and both IL-8 and PGE2 induce hyperalgesia.41–43
The combination of the abundant innervation of the disc and increased production of proinflammatory mediators suggests that discogenic pain may involve hyperalgesia.38 Injection of corticosteroids into the anterior epidural space has long been used to bathe the posterolateral periphery of the annulus to help curtail the biochemical stimulation of the intervertebral disc. The main goals of this approach are to improve pain and function and allow the patient to participate in a comprehensive physical therapy program addressing biomechanical deficiencies after this reduction of hyperalgesia is achieved. Before using ESIs, the target disc may be confirmed as the source of pain. To ensure the success of this approach, the appropriate therapeutic medication deposited into the anterior epidural space must also gain access to sensitized nerve endings.
The instillation of corticosteroid and anesthetic into the anterior epidural space introduces therapeutic agents with potent anti-inflammatory properties adjacent to suspected painful intervertebral discs. Local anesthetics help curtail inflammation by inhibiting phagocytosis, decreasing phagocytic oxygen consumption, reducing polymorphonuclear leukocyte lysosomal enzyme release, and diminishing superoxide anion production.44–48 Additionally, local anesthetics improve neural blood flow and function.49,50 Corticosteroids are well known for their anti-inflammatory properties, and also stabilize neural membranes, suppress ectopic neural discharges, and may have direct anesthetic effect on small unmyelinated nociceptive C-fibers.51–54 Painful lumbar intervertebral discs are innervated by substance-P containing nerve fibers, unmyelinated C-fibers, and thinly myelinated A delta fibers that provide a substrate on which corticosteroids and local anesthetics exert therapeutic benefit.41,42,55 The nucleus pulposus of the lumbar intervertebral disc is biologically active, responds to proinflammatory cytokines most sensitively after degeneration, and, once painful, produces further proinflammatory mediators.38,56 Hence, corticosteroids and local anesthetics may exert a therapeutic benefit by bathing the posterolateral annular fibers, which are most prone to injury, in solutions with anti-inflammatory and neural stabilizing effects.57–59
Indication
The primary indication for ESIs is CLBP with radicular pain. Despite minimal work having been completed investigating the efficacy of these interventions solely for axial lumbar spine pain, ESIs are routinely offered to patients presenting with axial CLBP presumed to be discogenic in origin based on a comprehensive evaluation of the patient.60 However, the role of ESIs to treat axial CLBP has not been well defined and is currently supported largely by conjecture and expert opinion. Deciding which level to inject is influenced by imaging findings and pain referral zones, but is more commonly determined by initially targeting the levels most likely to be responsible for discogenic CLBP (L4-5 and L5-S1).12,61 If the patient experiences only short-term or no improvement with S1 TFESIs adequately targeting the L4-5 and L5-S1 discs, lumbar discography would be warranted to determine whether one or both of the lowest two discs, or perhaps an upper level disc, is painful.
The ideal patient for ESIs would have CLBP with discogenic pain, provocative discography to demonstrate outer annular disruption causing the patient’s usual symptoms, and internally sound, nonpainful adjacent discs serving as internal controls. A reasonable algorithmic approach to a patient presenting with CLBP most consistent with a discogenic etiology is to perform TFESIs to empirically target the L4-5 and L5-S1 discs and monitor outcomes.61,62
Assessment
Before receiving ESIs, patients should first be assessed for LBP using an evidence-based and goal-oriented approach focused on the patient history and neurologic examination, as discussed in Chapter 3. Clinicians should also inquire about medication history to note prior hypersensitivity/allergy or adverse events (AEs) with drugs similar to those being considered, and evaluate contraindications for these types of drugs. Diagnostic imaging is often required before administering ESIs for CLBP. At minimum, plain film radiography of the lumbar spine is required to identify anatomic variations that may impact needle placement, as well as a general assessment of alignment, disc height, and stability.
In addition, advanced imaging such as MRI or CT can be used to help guide the spine specialist to target the appropriate spinal levels involved in a patient’s CLBP. Findings on advanced imaging that may influence the clinician’s recommendation of ESIs include degenerative endplate changes and high-intensity zone lesions, which may help to differentiate painful from nonpainful segments.63–65
Interventional diagnostic testing is also used occasionally before recommending or performing TFESIs, especially in cases of symptoms that persist for more than 6 months and prove recalcitrant to exhaustive conservative treatment measures. The use of provocative discography for CLBP remains controversial, but is used by some clinicians to identify painful annular fissures that may subsequently be targeted with other interventions such as ESIs. When interpreting provocative discography, extension of isotopic dye into or beyond the outer annulus has been shown to be the strongest predictor of concordant pain.61 Findings of concordantly painful outer annular disruption on discography would indicate to the clinician which discs may be targeted with TFESIs to achieve maximum impact. However, such a strategy has not been critically evaluated and further study is required.
Efficacy
Clinical Practice Guidelines
The CPG from Belgium in 2006 found low-quality and conflicting evidence to support the efficacy of ESIs for the management of CLBP with radicular pain.66 That CPG also found no evidence to support the efficacy of ESIs for the management of CLBP without radicular pain. There was low-quality evidence to support the efficacy of TFESIs for the management of CLBP with radicular pain.
The CPG from Europe in 2004 found conflicting evidence to support the efficacy of ESIs when compared with sham or other procedures for the management of CLBP with radicular pain.67 That CPG also found no evidence to support the efficacy of ESIs for the management of CLBP without radicular pain. ESIs for CLBP were not recommended.
The CPG from Italy in 2007 reported that ESIs could be considered as one management option for CLBP with radicular pain when adequate relief is not obtained with medication alone.68
The CPG from the United States in 2007 reported that ESIs could be considered as one management option for CLBP with radicular pain associated with a prolapsed disc when adequate relief is not obtained with other conservative interventions.69
The CPG from the United States in 2009 found poor evidence to support the efficacy of ESIs in the management of CLBP.70
The CPG from the United Kingdom in 2009 did not recommend ESIs for the management of CLBP.71
Findings from the above CPGs are summarized in Table 23-1.
TABLE 23-1 Clinical Practice Guideline Recommendations on Epidural Steroid Injections for Chronic Low Back Pain
| Reference | Country | Conclusion |
|---|---|---|
| 66 | Belgium | Low-quality and conflicting evidence of efficacy of ESIs and no evidence of efficacy of ESIs in CLBP without radicular pain Low-quality evidence to support efficacy of TFESIs in CLBP with radicular pain |
| 67 | Europe | Not recommended |
| 68 | Italy | Consider if adequate relief not obtained with medication |
| 69 | United States | Consider if adequate relief not obtained with other conservative interventions |
| 70 | United States | Poor evidence to support efficacy |
| 71 | United Kingdom | Not recommended |
CLBP, chronic low back pain; ESI, epidural steroid injection; TFESI, transforaminal epidural steroid injection.
Systematic Reviews
Cochrane Collaboration
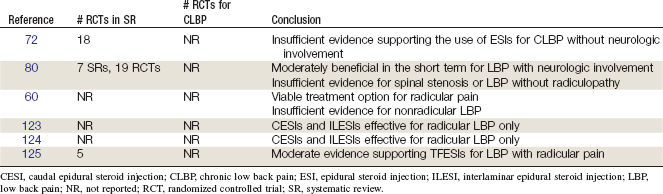
The Cochrane Collaboration conducted an SR in 2007 on injection therapy for subacute and chronic LBP without neurologic involvement.72 A total of 18 RCTs were identified, including RCTs related to ESIs for patients with LBP and neurologic involvement, LBP without neurologic involvement, failed back surgery syndrome, CLBP, and degenerative spinal disease.73–79 Two RCTs did not find any statistically significant differences between ESIs and placebo for pain, disability, and general improvement.73,74 In two RCTs of failed back surgery syndrome, no statistically significant differences were observed between ESIs and nonsteroidal anti-inflammatory drugs or morphine.76,77 In one RCT including LBP without neurologic involvement, no statistically significant differences were observed for ESIs versus intrathecal benzodiazepine for pain relief or general improvement.75 Two RCTs did not find any statistically significant differences between ESIs combined with ropivacaine versus ESIs combined with bupivacaine for CLBP or degenerative spinal disease.78,79 This review concluded that there is insufficient evidence supporting the use of ESIs for subacute and chronic LBP without neurologic involvement.72 This SR also recommended that future RCTs be conducted to determine patient subgroups who are most likely to benefit from ESIs.
American Pain Society and American College of Physicians
The American Pain Society (APS) and American College of Physicians (ACP) CPG committee conducted an SR in 2008 on nonsurgical therapies for acute and chronic LBP.80 That review identified seven SRs related to ESIs, one of which was the Cochrane Collaboration review mentioned earlier.72,81–86 Two high-quality SRs concluded that ESIs were not significantly different than placebo, yet another higher-quality SR concluded that ESIs were more effective at improving symptoms than placebo for LBP with neurologic involvement.72,82,86 In addition, two lower-quality SRs concluded that there was short-term pain relief from ESIs for LBP with neurologic involvement.81,85 The conclusions of two of the SRs were not reported.83,84 These SRs included 19 RCTs examining LBP with neurologic involvement of acute and subacute duration, mixed subacute and chronic, chronic, mixed duration LBP, and unknown duration.73,74,87–103 The rest of the RCTs examined LBP without neurologic involvement of unknown duration, including seven additional RCTs identified by the APS and ACP.1,24,75-77,104-122
RCTs comparing ESIs with nonepidural placebo injections for LBP with neurologic involvement generally found short-term benefits in pain and function (n = 5/6 RCTs). However, RCTs that compared ESIs with epidural placebo injections generally did not find any statistically significant improvement for LBP with neurologic involvement (n = 9/11). Three RCTs had unclear results for LBP with neurologic involvement. Long-term benefits for ESIs were not commonly observed among the RCTs (n = 4/18). ESIs were not consistently associated with lower rates of surgery versus placebo injections (n = 2/7). RCTs examining LBP without neurologic involvement, spinal stenosis, and failed back surgery did not find consistent benefits with ESIs. This SR concluded that ESIs are moderately beneficial in the short-term for LBP with neurologic involvement.80 It also concluded that there is insufficient evidence for ESIs among those with spinal stenosis or LBP without radiculopathy. The SR recommended that future RCTs should examine the long-term benefits of ESIs as well as compare ESIs with saline or local anesthetic versus a nonepidural placebo injections to determine the utility of ESIs.
Other
In 1994, a review was published by the Australian Working Party of the National Health and Medical Research Council summarizing recommendations for ESIs in the management of LBP.60 This review referenced a body of evidence endorsing ILESIs and CESIs as viable treatment options for LBP with radicular pain but cited a minimal body of literature evaluating the use of ESIs for nonradicular LBP.
Another review conducted a meta-analysis of RCTs which reported that both CESIs and ILESIs were effective for radicular, but not axial, LBP.123
A review identified 15 RCTs evaluating CESIs and ILESIs for LBP with or without sciatica.124 Although studies generally supported the use of ESIs in LBP with sciatica, there was no clear evidence supporting their use for CLBP without radicular pain. Review authors did not evaluate the clinical utility of the ESI techniques used by each study and were thus unable to comment on the possible effects of RCTs without fluoroscopic guidance and contrast confirmation of accurate needle placement.
A more recent review assessed the efficacy and safety of TFESIs and selective nerve root blocks for LBP with radiculopathy.125 That review was based on six clinical trials, including five RCTs and one OBS. It concluded that there was moderate evidence to support the use of TFESIs for LBP with radicular pain.
Findings from these SRs are summarized in Table 23-2.
Randomized Controlled Trials
Caudal Epidural Steroid Injections
Two RCTs were identified.74,126 Their methods are summarized in Table 23-3. Their results are briefly described here. The critical features of each study that must be assessed are the route of injection (fluoroscopic control), number of injections, clinical presentation (axial LBP vs. radicular pain), diagnostic evaluation (provocative discography), length of follow-up, and outcome measures.
TABLE 23-3 Randomized Controlled Trials of Caudal Epidural Steroid Injections for Chronic Low Back Pain
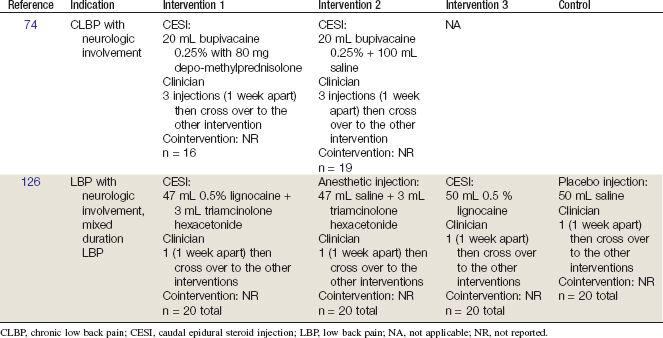
Breivik and colleagues74 in a prospective, double-blind, crossover study, assessed improvement in CLBP and sciatic pain in 35 patients treated with up to 3 blind, CESIs of either bupivicaine and methylprednisolone or bupivicaine and normal saline. The study followed a parallel, cohort design allowing patients not benefiting from their randomized treatment to then undergo treatment in the reciprocal arm. Initially, 56% of patients receiving methylprednisolone experienced significant relief compared with 26% treated with bupivicaine and saline. In the crossover, 14% of the methylprednisolone group obtained relief from subsequent bupivicaine and saline injections, whereas 73% of the bupivicaine and saline group reported satisfactory relief after the methylprednisolone injection. Fifty percent of the steroid group and 20% of the bupivicaine group returned to work at a range of 3 to 17 months after treatment. Up to three injections were performed in each arm. Thirty-two patients had undergone radiculography demonstrating disc prolapse, arachnoiditis, or inconclusive findings. However, the CESIs were performed without fluoroscopic guidance, and no further diagnostic testing had been conducted.
In a subsequent study, Yates performed caudal injections of saline, lidocaine, saline and triamcinolone, and lidocaine and triamcinolone in random order in 20 consecutive patients with LBP.126
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree