Chapter 1 Epidemiology and Etiology of Discogenic Pain
How Big Is the Problem?
 Provocative discography performed with controlled pressure manometry is currently the only definitive diagnostic test available.
Provocative discography performed with controlled pressure manometry is currently the only definitive diagnostic test available.Introduction
Discogenic pain is defined as pain originating from the intervertebral disc itself.1 It is nonradicular and may occur in the absence of spinal deformity, instability, and signs of neural tension.2 Although the external outline of the disc may remain intact, multiple processes (e.g., annular tears, degeneration, endplate injury, inflammation) can stimulate multiplication and possibly sensitization of pain nociceptors within the disc independent of nerve root symptoms. The concept of discogenic pain was introduced by Inman and Saunders in 1947,3 and the term was first used by Fernstrom in 1969 to establish the association between annulus stimulation and back pain perception identified during in vivo studies.4 Internal disc disorders were originally documented by Crock,5 who in 1970 was the first to study the mechanism of discogenic pain. He subsequently defined the term internal disc disruption (IDD) to describe unremitting lumbar spinal pain that lasted longer than 4 months, was unresponsive to conservative care, and could be reproduced with discography.6 Epidemiological studies suggest that cervical and lumbar discogenic pain reflect complex diseases (with “disease” defined as any impairment of normal physiological function of an organ or body part)7 that are accompanied by pathophysiological, biomechanical, psychological, and social implications. Such research offers insight regarding the magnitude of these diseases, their natural history, and the individual and external risk factors associated with each disease process. Even though 15% of the general population is reported to experience thoracic pain, the role of the thoracic disc as a source of chronic pain has not been well researched.8
The existence of a nerve supply to the disc and the concept of the intervertebral disc as an independent cause of spinal pain were controversial initially. Both are now well documented in the scientific literature and form the basis of current practice.9–24 Research by Nachemson, Bogduk, Aprill, Derby, and others has helped define the importance of this complex pain generator over four decades, as illustrated by the following quotes from their respective publications:
“Although practically all anatomic structures in the region of the motion segment have their proponents in the etiology of back pain, the lower intervertebral disc most likely causes the pain.”25 (Nachemson, 1976)
“These anatomical findings (i.e., cervical sinuvertebral nerves that supply the disc) provide the hitherto missing substrate for primary disc pain and the pain of provocation discography.”26 (Bogduk, Windsor, and Inglis, 1988)
“The outer third of the annulus fibrosis is richly innervated, and nerves may extend as deeply as the middle third of the annulus. This innervation constitutes the anatomic substrate for discogenic pain.”27 (Schwarzer and associates, 1995)
“Different, and independent, techniques point to the same conclusion. IDD has a distinctive morphology that correlates strongly with pain, and IDD has biophysical properties that correlate strongly with pain. For no other cause of low back pain have such multiple and strong correlations been demonstrated.”28 (Bogduk, 2005)
This chapter provides an overview of the epidemiology and etiology of discogenic pain.
Epidemiology
The epidemiology of cervical and lumbar spinal pain is quite impressive.29 At some time in their lives, an estimated 60% to 70% of the population will suffer from neck pain, and 65% to 80% of the population will suffer from low back pain.29–31 The annual prevalence of frequent or persistent neck and low back pain is 2% to 11% and 5% to 20%, respectively.32,33 In a 2008 survey of U.S. adults, 14% reported neck pain, and 26% reported low back pain within the previous 3 months.34 Cervical discogenic pain is estimated to be the cause of persistent neck pain in 16% to 41% of patients.35,36 Discogenic low back pain is cited as the most common cause of chronic low back pain, accounting for approximately 26% to 39% of its incidence.37,38
Low back pain is one of the most common health problems in the industrialized world.39,40 It is estimated that 28% of U.S. industrial workers will experience disabling low back pain at some time in their career and 8% of the entire working population will be disabled by low back pain in any given year, contributing to 40% of all lost work days. After adjustment for inflation, the total estimated medical costs associated with the treatment of neck and back pain is about $86 billion per year.41
Etiology
Introduction
The etiology of discogenic pain is as complex as the network of nerves surrounding it. When observing an anatomical dissection of the intervertebral disc, it is clear that its sensory innervation involves branches from the sinuvertebral nerve, anterior primary ramus, gray ramus, and sympathetic chain (Fig. 1-1). In addition, the work of Takahashi, Aoki, and Ohtori42 and Nakamura and associates43 has demonstrated that there may also be ascending pathways within the sympathetic chain to upper lumbar dorsal root ganglion neurons.
In a diseased disc, pain may be generated from deep within its own tissue. For years it was assumed that the nucleus, inner annulus, and mid annulus were completely avascular and aneural and that only the very outer layers of the posterior and anterolateral annulus contained nerve fibers. It is now clear that these pain-carrying nerve fibers can extend inward, deep into the middle annulus, and even into the nucleus in some cases. Not only has this been observed in degenerative discs, but it has also been linked to discogenic back pain (Figs. 1-2 and 1-3).44
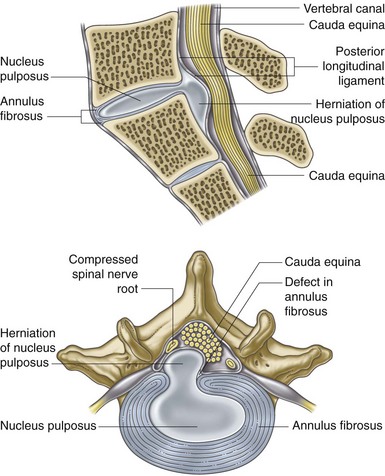
Fig. 1-2 Posterolateral disc prolapsed.
From Moore K, Agur AMR: Essential clinical anatomy, ed 2, Philadelphia, 2002, Lippincott Williams & Wilkins.
As early as 1997 Freemont and colleagues45 observed that 77% of discs that were surgically removed from patients with discography-positive discogenic pain had nerve fiber ingrowth into the middle one third of the annulus. Among the normal control discs, only 6% exhibited this pattern of ingrowth. These pain fibers were also associated with substance P, an active neural transmitter in pain transmission. Brisby46 identified peripheral sensitization, and thus amplification of the pain response in the sensitized disc, through the secretion of proinflammatory mediators. Increased numbers of mechanoreceptors and pain-producing neurons have also been confirmed both clinically and experimentally in the discs of patients with chronic discogenic pain.47–49 Given the somatosensory and autonomic neural innervation and the peripheral sensitization and amplification mechanisms impacting the pain response, it is understandable why the etiology and ultimate presentation of discogenic pain is so complex. In fact, as Mooney hypothesized in 1987,50 “there is something unique about the nerves related to the spine and the spinal canal, which makes the source of pain different from the rest of the musculoskeletal parts of the body.” He suggested that the disc is uniquely innervated with a predominantly visceral-type nerve supply.
Normal Disc Physiology
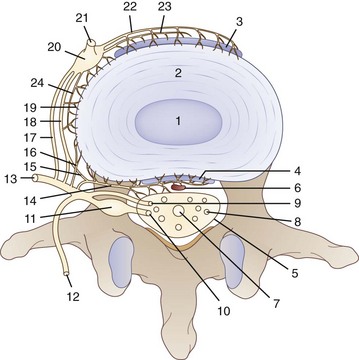
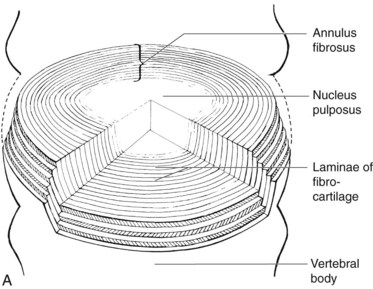
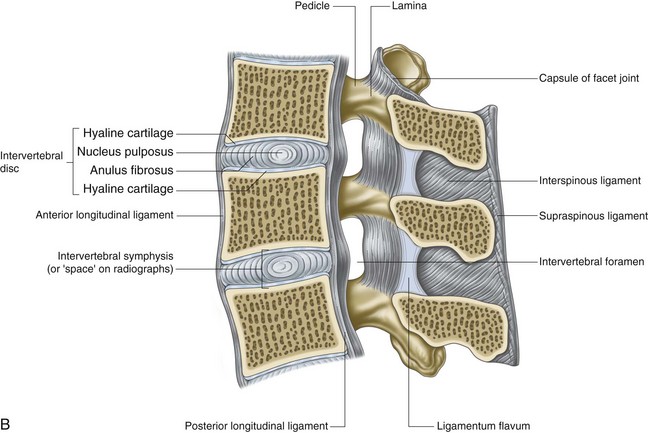
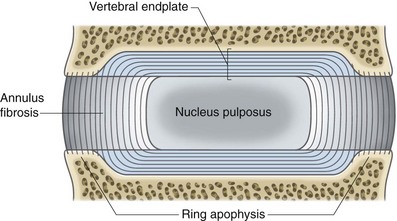
To understand the etiology of discogenic pain, it is helpful to first understand the basic physiology of the normal intervertebral disc. Discs lie between each of the vertebral bodies and link them together (Fig. 1-4). In total the discs comprise 25% of the height of the spinal column.51 In the lumbar region discs are approximately 7 to 10 mm thick and 4 cm in diameter.52,53 The discs function to resist spinal compression and evenly spread the load on the vertebral bodies while allowing limited movements. The intervertebral disc is comprised of three distinct regions: the nucleus pulposus, the annulus fibrosus, and the endplate (Figs. 1-5 and 1-6).54 The vertebral endplate is considered a component of the intervertebral disc rather than a part of the vertebral body. The endplates are not attached to the subchondral bone of the vertebrae; they are instead strongly interwoven into the annulus of the disc and are therefore considered part of this anatomic structure.55 The attachment of endplate to vertebral body is less robust and is susceptible to injury.56 The nucleus pulposus is the gelatinous core of the disc and is normally aneural and avascular.57 Its matrix is maintained by chondrocyte-like cells, which are stimulated by growth factors to produce constituents, including elastin, type 2 collagen, and proteoglycans.58 Proteoglycans consist of a core central protein from which chains of glycosaminoglycans (GAGs) project. Multiple proteoglycans are joined by a hyaluronic acid chain to form aggrecan, which is highly hydrophilic. The resulting hydroscopic and hydrostatic properties of the nucleus pulposus are the factors that allow it to accommodate compression.59,60 Nucleus pulposus cells also inhibit the enzymes responsible for matrix breakdown (e.g., matrix metalloproteinases).61 Matrix constituents are not static; they continually degrade the matrix and provide it with newly synthesized components.
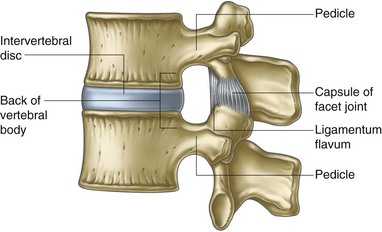
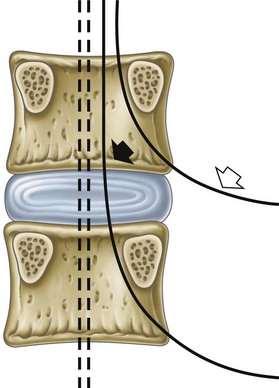
Fig. 1-4 Boundaries of an intervertebral foramen.
From Standring et al, editors: Gray’s anatomy: the anatomical basis of clinical practice, ed 39, 2005, Elsevier Churchill Livingstone, p 742.
The nucleus pulposus is encircled by the annulus fibrosus, which is composed of a series of 15 to 25 concentric rings of type 1 collagen that resist pressure from the gelatinous core of the disc.62 The cells in the outer annulus fibrosus are fibroblast-like, elongated, and thin; and they lie parallel to the collagen fibers. The cells in the inner annulus tend to be more ovular.63 Innervation of the healthy annulus fibrosus is primarily restricted to the outer lamellae.64 The posterior annulus and the adhering longitudinal ligament are supplied by the sinuvertebral nerve, which is a mixed autonomic and somatic nerve. The anterior and lateral annuli are supplied by autonomic nerves.65
Normal Disc Aging and Degeneration
The most common and striking feature of disc aging and degeneration is the loss of the proteoglycan molecule from the nucleus of the disc. Other signs of aging include progressive dehydration, progressive collagen thickening caused by cross linking and glycation, formation of brown pigmentation, and increased “brittleness” of the disc tissue.66 These changes are all amplified by the naturally poor vascular supply of the disc via the capillary beds just above the vertebral endplates (Fig. 1-7).67
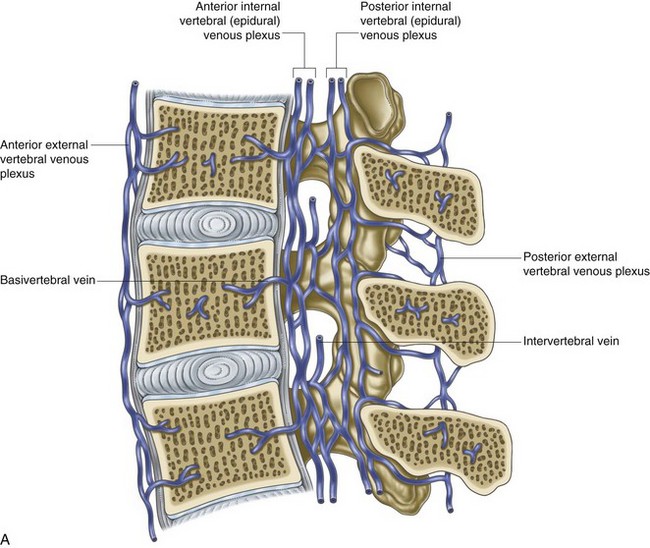
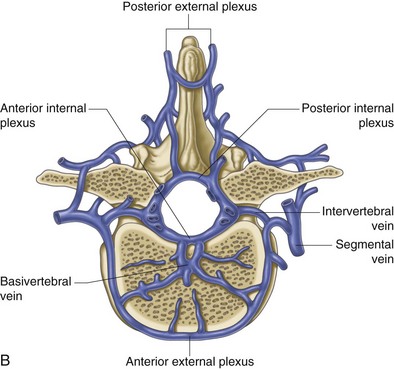
Fig. 1-7 A and B, Venous drainage of the vertebral column.
From Standring et al, editors: Gray’s anatomy: the anatomical basis of clinical practice, ed 39, 2005, Elsevier Churchill Livingstone, p 739.
The degenerative cascade, described by Kirkaldy-Willis and associates,68 is a widely accepted pathophysiological model for describing the degenerative process as it affects the lumbar spine and individual motion segments. The process occurs in three phases that comprise a continuum with gradual transition rather than three clearly defined stages. Phase 1 (dysfunctional) is characterized histologically by circumferential tears or fissures in the outer annulus. Tears can be accompanied by endplate separation or failure, blood supply interruption to the disc, and impaired nutritional supply and waste removal. Such changes may be the result of repetitive microtrauma. Since the outer one third of the annular wall is innervated, tears or fissures in the area may lead to discogenic pain. As the disc progresses through phase 2 (unstable) and phase 3 (stabilization), biochemical changes occur that can lead to chronic IDD and disc herniations.
In 2002 Boos and colleagues69 won the Volvo Award in Basic Science for their observations regarding an idiopathic “obliteration” of parts of the nutrient-providing capillary beds lying just above the vertebral endplates. This auto-destruction begins within the first 2 years of life and increases over the next 8 years. These findings suggest that the initial cause of disc aging and degeneration is “nutritional compromise,” which is secondary to the loss of the discal blood supply above the vertebral endplates.
It is not clear why the nucleus of the disc loses much of its vital blood supply during the first decade of life.70 Without sufficient nutrients (which are transported in the blood) the cells of the disc begin to die, and the disc becomes depleted of water.71 This correlates with the initial appearance of radial tears seen during the second decade of life, which is also the age when the first signs of discogenic low back pain may occur.70
These observations were substantiated by Horner and Urban’s Volvo Award–winning study of the viability of living human disc cells under varying conditions.72 They concluded that, if the cells of the disc failed to get proper nutrients (e.g., oxygen or glucose) or if the pH level of the disc changed (i.e., as a result of increased lactic acid or waste not being diffused out of the disc), its cellular metabolism would be directly affected. More specifically, the disc would stop producing the vital proteoglycan molecules. Proteoglycans are made of a protein core attached to at least one GAG chain. The predominant GAGs in the intervertebral disc are chondroitin-6-sulfate and keratin sulfate. These proteoglycans combine within the disc to produce large-aggregate molecules.73 Without proteoglycans the disc dehydrates and loses its hydrostatic and osmotic pressure. Several experimental studies have concluded that intervertebral disc cells are very sensitive to the amount of hydrostatic pressure under which they can function.74,75 Discs perform optimally at approximately 3 atm (44 psi), which is the normal pressure observed in a healthy disc. Any pressure variation either above normal or especially below 1 atm (14.6 psi) impairs disc function. The loss of the proteoglycan content is the most striking feature of disc aging, degeneration, death, and the development of annular tears.76 Trout, Buckwalter, and Moore77 confirmed that by adulthood more than 50% of the cells of the disc were dead.
Collagen is the other major structural component of the extracellular matrix of the intervertebral disc (Fig. 1-8). It provides tensile strength, allows for stability between vertebrae, and resists excessive disc bulging in response to loads. In younger discs, collagen comprises 67% of the dry weight of the annulus fibrosus and 25% of the nucleus pulposus. There are many types of collagen within the disc; however, types 1 and 2 comprise 80% of the total collagen content of the disc. Type 1 collagen is most abundant within the annulus, and type 2 comprises 80% of the collagen within the nucleus.73 The loss of optimal intradiscal vascular supply and thus nutrition results in glycation, which is a biochemical reaction during which sugars come in contact with proteins (such as disc collagen) in an avascular and low-oxygen environment. This reaction converts the collagen strands, which are highly stable and have high tensile strength, into bulky, brittle, fibrous-type tissue that is more susceptible to degeneration and annular tears.73,78 When stained, this tissue is a distinct shade of brown, as noted previously regarding the development of brown pigmentation in the aging disc. These physiological changes result in an intervertebral disc that is more susceptible to injury and possibly the development of discogenic pain.
Over the last decade significant research in the field of nuclear magnetic resonance spectroscopy has enhanced our understanding about the relationship between metabolic biochemical markers and discogenic low back pain.79–81
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree