Environmental Hazards
Daniel Aronzon MD, FAAP
Pradeep Sharma MD
Richard Bachur MD
Sunil K. Sood MD
Vincent P. Beltrani MD
PART I Lead Poisoning
Daniel Aronzon MD
INTRODUCTION
In the early 1970s, lead poisoning was viewed as an environmental hazard limited to large, urban pediatric inpatient services, where occasionally children with encephalopathy were diagnosed and treated. It was falsely assumed that only such high-level exposures caused permanent damage, and studies focused on the effects of chelation therapy for very high blood lead levels (BLLs) in excess of 45 mcg/dL and its ability to reverse deleterious neurologic effects (Silbergeld, 1997).
Newer studies raised the very disturbing concern that lead could cause irreversible damage at much lower BLLs than previously imagined. A landmark article by Needleman and Gatsonis (1990) proved that exposure to lead had significant effects on the behavior and cognition of children. Clearly, chelation therapy can lower BLLs and total body lead, and in doing so, it can prevent acute neurotoxicity, such as seizures. What is unclear is whether postexposure chelation can reverse the long-term neurologic sequelae of lead poisoning (Silbergeld, 1997).
These concerns have led to a new public health strategy focusing on eliminating lead exposure from the environment and recognizing that toxicity may be associated with a BLL above 10 mcg/dL. National initiatives in the United States have eliminated lead from gasoline and indoor paints. The variables of income, race, and residence, however, and the fact that lead poisoning is not evenly distributed among all children in the United States continue to raise obstacles to its universal prevention (Silbergeld, 1997).
PATHOLOGY
The classic markers of lead toxicity, encephalopathy, and red blood cell basophilic stippling are rarely seen in this era (American Academy of Pediatrics [AAP], 1995). Numerous studies have demonstrated the relationship between BLLs and cognitive defects. The 1998 AAP Policy Statement lists cognitive deficits, attention problems, and aggressive, antisocial, and delinquent behaviors as possible sequelae of low-level lead poisoning. It also provides an excellent bibliography.
For every 10 mcg/dL increase in BLL, there is an estimated decrease of two IQ points (Glotzer & Weitzman, 1995).
Diet affects lead toxicity. Iron deficiency increases the absorption of lead from the gastrointestinal (GI) tract, while adequate iron intake decreases absorption. Calcium deficiency increases lead retention in bone.
Knowledge of lead pharmacokinetics is very primitive. Elimination from bone is measured in years. BLLs represent only a fraction of the total body lead, the majority of which is in bone. Chelation therapy temporarily lowers BLLs until equilibration with bone takes place. If any reduction in total body lead is to occur, it may require long-term chronic chelation therapy, the effects of which are unknown (AAP, 1995).
EPIDEMIOLOGY
The mean BBL in the United States has decreased from 12.8 mcg/dL in 1980 to 2.3 mcg/dL in 1994. In 1994, 2.2% of the population had BLLs greater than 10 mcg/dL. From 1980 to 1994, the percentage of children with BLLs greater than 10 mcg/dL decreased from 88.2% to 4.4%. Figures are much higher, however, among African American, poor, and urban children. These declines are attributed to the removal of lead from gasoline, paint, and food cans. Lead in paint was banned in 1970, and lead-containing gasoline was gradually phased out and finally banned in 1990 (AAP, 1998; Needleman, 1998).
Lead poisoning is not just a problem for the urban poor. Seventy-four percent of privately owned homes contain lead paint, with age and condition of housing, not location, as prime risk factors (AAP, 1998). Lead-based paint in the form of paint chips and house dust are the prime sources. Other sources include soil (contaminated from gasoline for decades), water (from lead pipes), household renovations, occupational exposures, and lead-containing pottery, folk medicines, and cosmetics.
HISTORY AND PHYSICAL EXAMINATION
Low-level chronic lead poisoning may not be associated with any specific clinical findings other than a history of older housing. It is associated with a hypochromic microcytic anemia, and lead exposure should be considered in that differential.
Acute intoxication may result in abdominal pain, constipation, headaches, vomiting, and in severe cases, changes in the level of consciousness or seizures.
DIAGNOSTIC CRITERIA AND STUDIES
The diagnosis is commonly established from the results of screening tests. Risks for lead intoxication are determined from patient questionnaires and actual measurement of BLL.
MANAGEMENT
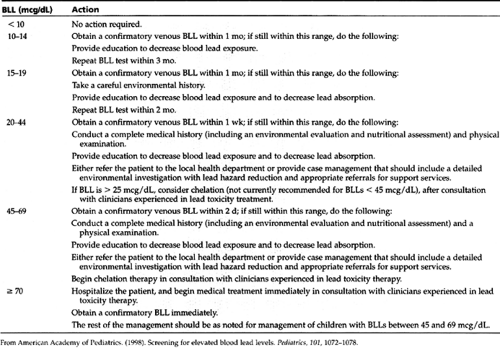
Management falls into four basic categories: identification through screening; patient education; abatement; and chelation for those with elevated levels. Screening and chelation are discussed in the following sections.
Screening
In 1991, the Centers for Disease Control and Prevention (CDC) recommended and the AAP endorsed universal screening between ages 9 and 12 months and then again at age 2 years. They revised these guidelines in 1998, because in certain geographic areas, the incidence of abnormal BLLs was so low that it did not justify the cost. The CDC felt that targeted screenings would be more appropriate than universal screenings. The AAP endorsed and justified this measure to reduce unnecessary testing of children unlikely to be at risk.
The new guidelines appear complex and difficult to interpret and implement. In communities where the prevalence of elevated BLL (>10 mcg/dL) is unknown and where older housing predominates (more than 27% built before 1950), universal screening is still recommended. Targeted screening based on the answers to a questionnaire (Display 48-1) is recommended for locales where less than 12% of children have BLLs greater than 10 mcg/dL or where less than 27% of the homes were built before 1950. The burden for determining which communities will have targeted or universal screening falls to local and state public health officials (AAP, 1998). It is not surprising that this apparent backslide in policy has raised controversy, particularly among public health officials (Needleman, 1998; Silbergeld, 1997).
DISPLAY 48–1 • A Basic Personal-Risk Questionnaire*
|
The state or local health department may recommend alternative or additional questions based on local conditions. If the answers to the questions are “no,” a screening test is not required, although the provider should explain why the questions were asked to reinforce anticipatory guidance. If the answer to any question is “yes” or “not sure,” a screening test should be considered.
From American Academy of Pediatrics. (1998). Screening for elevated blood lead levels. Pediatrics, 101, 1072–1078.
Patient education is recommended as part of anticipatory guidance for all. The AAP in its 1998 policy statement identifies risk factors and prevention strategies that are listed in Table 48-1. Management of elevated BLLs identified during screening, as recommended by the AAP, including education and abatement, is outlined in Table 48-2.
Fingerstick lead results may be falsely high due to dust contamination. No treatment decisions can be made without first obtaining a confirmatory venous sample.
Chelation
The 1995 AAP policy statement provides an excellent review of the classic chelation modalities, dimercaprol (BAL in oil) and calcium disodium EDTA. Parenteral administration for each is required. Penicillamine is also discussed as an oral chelator.
Most clinicians who treat lead poisoning now use succimer (an oral analog of dimercaprol). As an oral agent, it may be used on an outpatient basis but only after environmental safety is guaranteed. There is no evidence that with BLLs between 25 and 45 mcg/dL, chelation avoids or reverses neurotoxicity (Berlin, 1997); however, if elevated levels persist in this range, despite all efforts at abatement, succimer may be indicated after consultation with an expert. Succimer is definitely indicated for children with BLLs greater than 45 mcg/dL who have no symptoms of encephalopathy. Hospitalization
is usually recommended, initially to monitor for side effects and then either to abate the current housing or to find new safe housing. The dose is 30 mg/kg/d for 5 days followed by 20 mg/kg/d for 14 days. Some children report GI discomfort and malaise. There have been case reports of reversible neutropenia and anemia; therefore, hematologic surveillance is recommended during therapy (AAP, 1995).
is usually recommended, initially to monitor for side effects and then either to abate the current housing or to find new safe housing. The dose is 30 mg/kg/d for 5 days followed by 20 mg/kg/d for 14 days. Some children report GI discomfort and malaise. There have been case reports of reversible neutropenia and anemia; therefore, hematologic surveillance is recommended during therapy (AAP, 1995).
For cases of encephalopathy manifested by changes in the level of consciousness, headache, or vomiting or those with BLLs greater than 70 mcg/dL, parenteral chelation combining BAL and EDTA is advised. Only professionals experienced in treating lead toxicity should undertake this type of treatment.
• Clinical Pearl
After completion of chelation therapy, the provider should obtain a repeat venous lead level after 14 days to allow for reequilibration. Further management as outlined in Table 48-2 will be predicated on the repeat level.
REFERENCES
American Academy of Pediatrics Policy Statement. (1998). Screening for elevated blood lead levels. Pediatrics, 101, 1072–1078.
American Academy of Pediatrics Policy Statement. (1995). Treatment guidelines for lead exposure in children. Pediatrics, 96, 155–160.
Berlin, C. M. (1997). Lead poisoning in children. Current Opinion in Pediatrics, 9, 173–177.
Glotzer, T. E., & Weitzman, M. (1995). Commonly asked questions about childhood lead poisoning. Pediatric Annals, 24, 630–639.
Needleman, H. L., & Gatsonis, G. A. (1990). Low level lead exposure and the IQ of children: A meta-analysis of modern studies. Journal of the American Medical Association, 263, 673–678.
Needleman, H. L. (1998). Childhood lead poisoning: The promise and abandonment of primary prevention. American Journal of Public Health, 88, 1871–1877.
Silbergeld, E. K. (1997). Preventing lead poisoning in children. Annual Review Public Health, 18, 187–210.
PART II Allergies, Anaphylaxis, Stinging Insects (Hymenoptera), and Drug Allergies
Pradeep Sharma MD
Richard Bachur MD
ALLERGIES
Allergic diseases affect approximately 25% of all children. The economic implications are enormous, with direct medical costs approaching $1.2 billion and indirect costs estimated to be the same. Inclusion of diseases such as asthma, chronic sinusitis, otitis media with effusion, and nasal polyps, all associated with allergic rhinitis, bring the cost close to a staggering $10 billion. The incidence of allergic diseases, especially asthma, is rising; the exact causes for this increase are unknown. The exact incidence of anaphylaxis and acute allergic reactions is also unknown.
Upon presentation of an antigen, the immunologic response can proceed along two pathways: TH1 pathway (T helper cell 1) in the nonatopic individual and TH2 pathway in the atopic. In the nonatopic individual, exposure to antigens leads to the division of the helper T cells. The immune response then proceeds along the TH1 cell pathway, which, under influence of interluekin 2 and inteleukin 6, causes the B cell and plasma cells to produce immunoglobulin G (IgG) antibodies. These antibodies are protective and do not lead to an allergic reaction. In the atopic individual, however, the TH2 cell is involved, which secretes interleukin 4 (IL4), causing the B cell and plasma cells to secrete primarily IgE antibody. The IgE antibody then can affix to receptors on the mast cells.
Repeated exposure to antigens to which the host previously has been sensitized will then lead to degranulation of the mast cell, resulting in the release of preformed mediators of allergy, primarily histamine and heparin. The result is an immediate type 1 allergic reaction with clinical symptoms that can culminate in anaphylaxis. Also, mast cell degranulation releases several chemotactic factors that lead to a migration of inflammatory cells (eosinophils, neutrophils), leading to chronic airway inflammation and tissue injury, which are hallmarks of the late-phase reaction. The result may be chronic inflammation, such as seen in bronchial asthma and allergic rhinitis. The mast cell also is involved in activation of phospholipase A2, leading to activation of the arachidonic acid into leukotreines and prostaglandins.
One of the many approaches in treating allergic diseases includes drugs that lower IL4. Such drugs would reduce production of IgE antibodies and modify the immune response so that it proceeds along the TH1 pathway. Recent evidence has shown that one of the mechanisms by which allergy immunotherapy works may be by reduction in IL4 (Ebner, 1998).
ANAPHYLAXIS
Anaphylaxis is the acute onset of potentially life-threatening, multiorgan symptoms due to the release of mediators from mast cells and basophils. It occurs in individuals who are exposed to an antigen to which they were previously sensitized.
Pathology
The bridging of two adjacent IgE antibodies by an antigen leads to a release of preformed mediators of allergy, resulting in the early phase of the type 1 reaction and, in severe cases, anaphylaxis.
Reactions Based on the Immune System
IgE mediated (type 1) reactions are responsible for most cases of anaphylaxis that involve immune mechanisms. Common responsible agents are foods such as peanuts, milk, eggs, chocolate, and shellfish. Reactions to insect stings from Hymenoptera, vaccines, and allergy immunotherapy are also seen in children.
The following can cause anaphylaxis by IgE mediated immune mechanisms:
Antibiotics (haptens): penicillin, cephalosporins
Venomous (Hymenoptera): yellow jackets, white face hornets, yellow hornets, honey bee, and wasps
Foods: milk, peanuts, eggs, shellfish, chocolate
Vaccines: allergen extracts, tetanus
Hormones: insulin
Miscellaneous: latex
Antiserum: tetanus
Anaphylaxis by way of the immune system but not involving the IgE antibody would include anaphylaxis following a blood transfusion, which is usually a result of IgG-based immune complex mechanism. A complement-mediated anaphylaxis reaction can result from blood transfusion reaction associated with IgA deficiency.
Anaphylaxis from Nonimmune Mechanisms (Anaphylactoid)
The following are examples of anyphylaxis caused by nonimmune mechanisms:
Direct histamine-releasing agents: radiologic contrast media, succinylcholine, and ciprofloxin
Arachidonate-mediated: aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs)
Unknown mechanisms: exercise-induced anaphylaxis, cold urticaria, and mastocytosis
Epidemiology
The exact incidence of anaphylaxis in children is unknown; however, data suggest that the incidence of anaphylaxis may be 0.25% in patients receiving allergy immunotherapy. Anaphylaxis to Hymenoptera occurs in about 1% to 2% of the general population, and fatalities (mostly in adults) from Hymenoptera stings total approximately 25 to 50 per year in the United States. Fatal reactions to penicillin also have been reported but are extremely rare and occur mostly due to parenteral administration at a rate of 0.002%. Anaphylaxis is much less likely if penicillin is administered orally. The incidence
of fatal anaphylaxis appears to be twice as high in patients with asthma. Patients on beta-blockers also may be at high risk for anaphylaxis (Lang, 1995).
of fatal anaphylaxis appears to be twice as high in patients with asthma. Patients on beta-blockers also may be at high risk for anaphylaxis (Lang, 1995).
Risk factors for anaphylaxis include the following:
Asthma
Female sex
Nature of specific antigens; some allergens, such as those found in peanuts, shellfish, and penicillin, are much more likely to cause anaphylaxis than others.
Route of entry of allergen; intramuscular or intravenous (IV) route of entry is more likely to lead to anaphylaxis than the oral route.
Use of beta-blockers
History and Physical Examination
Quick recognition of clinical symptoms listed in Table 48-3 is key to establishing a diagnosis of anaphylaxis. Key features include rapid onset of generalized itching, urticaria, angioedema, flush, erythema, headache, sneezing, rhinorrhea, wheezing, difficulty swallowing, abdominal cramping, diarrhea, sensation of impending doom, respiratory arrest, and sometimes cardiovascular collapse. The presentation varies, and not all organ systems are involved in all patients. Nonetheless, in this potentially life-threatening situation, symptoms can start within seconds, reaching a peak severity from 5 to 30 minutes after exposure to the offending agent. Late-phase reaction also may occur 6 to 12 hours later, with symptoms lasting up to as much as 24 hours despite treatment. Most children have cutaneous symptoms, which may progress to involve the respiratory system and a feeling of anxiety. Only rarely, however, do symptoms progress to respiratory failure or cardiovascular collapse.
Diagnostic Criteria
The diagnosis is based on obtaining a history of exposure to an offending agent or recognition of the classic signs and symptoms. The presence of cutaneous symptoms and signs (ie, pruritus, urticaria, angioedema) makes the diagnosis more obvious.
Anaphylaxis can be confused with vasovagal syncope. Distinguishing features are that vasovagal reactions occur during stressful conditions, such as venipuncture, vaccinations, and allergy skin tests. Patients appear pale and sweaty and may have bradycardia. Unlike with anaphylaxis, patients with vasovagal episodes maintain normal blood pressure and have no pruritus, urticaria, angioedema, or bronchospasm. They also recover spontaneously within minutes.
Diagnostic Studies
Laboratory tests are generally not necessary to establish a diagnosis of anaphylaxis; however, tryptase levels are elevated in most cases. Providers may draw a level if diagnosis of anaphylaxis is in doubt. The window in which tryptase levels may accurately reflect anaphylaxis is about 2 hours after onset of symptoms (Schwart et al., 1989). After the event, if the etiology is in doubt, allergy skin testing may be indicated if a clinician suspects an ingestant (food), Hymenoptera sting, or inhalant as the culprit.
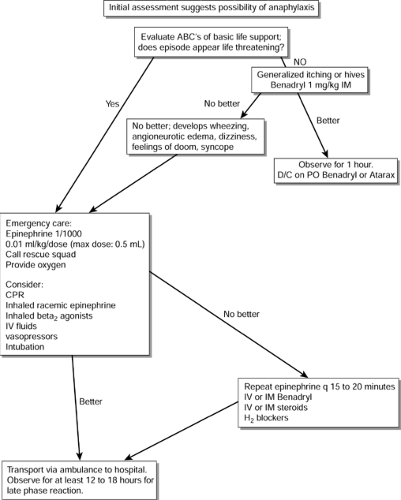
Management
Prompt recognition and treatment of anaphylaxis are important, because anaphylaxis can be life threatening. The reader is referred to the algorithm in Figure 48-1, which describes the treatment of an acute allergic reaction in the office or clinic setting. Epinephrine is the drug of choice in anaphylaxis. It reduces mediator release and may reverse the physiologic effects of many mediators. Because most providers in private practice are not experienced in advanced life support, which may be needed in management of anaphylaxis, they must know when to call for help for patients who fail to respond to the administration of epinephrine and antihistamines. Providers can encounter anaphylaxis at any time; therefore, mock drills practicing management of anaphylaxis may be helpful. An emergency cart accessible to all personnel also is essential. All health care providers should maintain basic cardiopulmonary resuscitation certification. Display 48-2 presents the basic supplies and equipment recommended for management of anaphylaxis in an office setting.
DISPLAY 48–2 • Basic Office Equipment for Managing Anaphylaxis
Stethoscope and sphygmomanometer
Tourniquets, syringes, hyperdermic needles, and a large-bore (14 gauge) needle
Aqueous epinephrine 1:1000
Equipment for administering oxygen by mask; make sure pediatric sizes are available
Equipment for administering intravenous fluids
Oral airway
Diphenhydramine or similar antihistamine
Coticosteroids for intravenous injections
Vasopressors
Instructions to School Personnel
Families should provide detailed written instructions to school personnel regarding management of anaphylaxis in individuals in whom anaphylaxis is likely. These instructions can be created from the contents presented in Display 48-3.
DISPLAY 48–3 • Emergency Health Care Plan
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Reactions to Egg-Based Vaccines
Clinicians often are confronted with a patient due to receive an egg-based vaccine (eg, measles, mumps, rubella (MMR); influenza; yellow fever) but is also allergic to eggs. Most reactions to MMR and other egg-based vaccines are not due to sensitivity to egg protein but to other vaccine constituents. In the case of MMR, when immediate allergic reactions do occur, most appear to be due to the gelatin or neomycin also present in the vaccine.
The MMR vaccine is a highly refined product prepared in chick embryo fibroblast cells, decreasing the amount of protein that could react with ovalbumin to a level unlikely to cause anaphylaxis. The influenza and yellow fever vaccines are prepared in embryonated cells and hence may contain higher amounts of egg protein.
Numerous studies have established the safety of MMR vaccine in egg-sensitive subjects. In reviewing 17 studies, MMR vaccine was safely administered to 1209 patients who were egg sensitive on skin test. Studies show that 99.75% of egg-sensitive patients with positive skin test to eggs received MMR vaccine without having an anaphylactic reaction.
• Clinical Pearl
The latest AAP recommendations in the 1997 Red Book state that children with a history of egg allergy or sensitivity may be given MMR without prior skin testing, because skin testing is not predictive of a vaccine-allergic reaction.
Egg-sensitive patients also have been given yellow fever vaccine safely. The data for influenza vaccine, however, are incomplete. Providers should use caution when administering influenza vaccine to egg-sensitive patients (Fasano, Wood, Cook, & Samoson, 1992).
The following are recommendations for administering yellow fever or influenza vaccines to egg-sensitive patients:
Take a detailed history. Does the child have a history of anaphylactic reaction to eggs, or was the reaction mild and localized to the skin?
Children or adolescents with less severe reactions to eggs may receive influenza or yellow fever vaccines and do not warrant skin testing.
Children with severe or anaphylactic reactions to eggs should not receive influenza vaccine, given the availability of chemoprophylaxis.
A trained allergist should perform skin testing on children with severe or anaphylactic reactions to eggs before administration of yellow fever vaccine.
HYMENOPTERA STINGS
The incidence of Hymenoptera sensitivity is about 2% to 3% of the general population. Young males tend to be stung more often, but most fatalities occur in the elderly. Fatal reactions also have been reported in the pediatric population.
History and Physical Examination
Most children report a painful sting, although they are unable to identify the insect that stung them. In the United States, the most common culprits are insects from the hornet family, with yellow jackets being most common, followed by other hornets, wasps, and finally honeybees. The various presentations consist of local and systemic reactions. At the least, there is usually redness, pain, and some degree of swelling at the site of the sting, due to irritation from the venom. Allergic reactions to Hymenoptera are sometimes confused with large local reactions, which are raised, red, painful, and at times itchy at the site of the sting. The differential point is that these reactions are limited to the bite site, and no systemic signs of generalized pruritus, urticaria, or airway difficulty are present. Local reactions do not involve the IgE antibody. Evidence has shown that about 50% of individuals with history of a systemic reaction give a history of progressively increasing large local reactions before occurrence of the first systemic reaction. Some individuals with large local reactions may have higher than normal IgE antibodies against the class of stinging insect responsible for the sting. The symptoms of more systemic reactions and anaphylaxis to a Hymenoptera sting are the same as for other causes, and the reader is referred to Table 48-3.
Diagnostic Criteria
The history of a sting immediately preceding a local, systemic, or anaphylactic reaction establishes the diagnosis. As recurrent allergic reactions to Hymenoptera insects can be life threatening, the provider needs to identify the stinging insect responsible. To do so, a consultation with an allergist for appropriate skin testing for Hymenoptera may be necessary.
Management
Acute Management
Bees tend to deposit a barbed stinger in the skin, whereas wasps can sting multiple times. If the stinger is present in the skin, removal should be as soon as possible. Although “scraping” the stinger was previously felt to be advantageous over using a forceps (which may squeeze more venom into the skin), a recent study did not find a difference related to removal method (Visscher, Veller, & Camazine, 1996).
Further treatment is based on the severity of the allergic reaction. Providers may treat local irritation or local hives initially with cold compresses. Patients exhibiting generalized itching or hives should receive diphenhydramine (Benadryl) 1 mg/kg (maximum 50 mg). They require observation for at least 1 hour. These patients can then be discharged on diphenhydramine or hydroxyzine (Atarax) for the next 24 hours. Patients with any other systemic reactions, including wheezing, angioneurotic edema, dizziness, vomiting, or syncope, should receive epinephrine 1:1000 (0.01 mL/kg, maximum 0.5 mL) subcutaneously. They then require transport by emergency personnel to an emergency room.
Patients with a previous history of anaphylaxis should be empirically treated with diphenhydramine 1 mg/kg and transported immediately to an emergency room by emergency personnel. These individuals should receive epinephrine (as above) for any signs of allergic reaction.
Follow-up Management
Local reactions require no follow up. If the reaction was systemic, the clinician should prescribe an antihistamine and epinephrine (an Epipen) for emergency use in the event of another sting and provide the patient or parents with specific written instructions regarding their use. For systemic reactions, the provider also should refer the child to an allergist. Following consultation with an allergist, if the history and workup (allergy skin tests for Hymenoptera) confirm allergy, then the provider, patient, and family should take the following measures:
Reduce sting possibility by avoiding bright-colored clothing, perfumes, colognes, and areas where Hymenoptera are common.
Manage accidental field stings by administration of oral diphenhydramine and use of epinephrine (Epipen or Epipen Jr.) as described in the module on anaphylaxis.
Use venom immunotherapy as appropriate. Venom immunotherapy for Hymenoptera is highly effective, with treatment failures occurring in less than 5% of patients. Data also suggest that protection from Hymenoptera stings may last for a long time after cessation of immunotherapy (Golden, Kwiterovich, Kagey-Sobotka, Valentine, & Lichtenstein, 1996). An experienced allergist should perform venom inmmunotherapy. It is indicated for children who have suffered from a life-threatening reaction, such as symptoms involving the airway or cardiovascular system. Children with strictly a systemic cutaneous reaction, such as urticaria, may be managed without venom immunotherapy. Exceptions may be made if the family desires the added protection provided by immunotherapy or if the patient is at a high risk for future stings because of special situations. Examples are a child allergic to honey bees whose parents are beekeepers or children who spend an unusual amount of time outdoors camping and hiking.
DRUG ALLERGY
Drug allergy is defined as an adverse event following administration of a drug due to a specific immunologic sensitization that usually involves the IgE antibody. This reaction must be distinguished from nonimmunologic events, such as side effects, toxic reactions, and drug-to-drug interactions.
Epidemiology
The exact incidence of drug allergy in children is unknown; however, about 5% to 15% of the general population will give a history of an adverse drug reaction as a result of penicillin. A controlled study suggests an actual incidence of 3.2%. Allergic reactions to second- or third-generation cephalosporins are rare (1%–3%). Cross-reactivity with patients who are allergic to penicillin is about 2%. The vast majority of parents confuse side effects and idiosyncratic reactions with true allergic reactions.
• Clinical Pearl
Only a small fraction of patients in whom a history of penicillin allergy is elicited actually have a true penicillin allergy.
History and Physical Examination
A meticulous history is most important and must include a detailed drug history. What medications was the child taking at the time of the reaction? Was the reaction urticarial? Was the rash pruritic? Were there signs of anaphylaxis? Unfortunately, in children, the history may not be reliable. Many parents or children do not remember the name of the antibiotic or type of reaction. Because the actual incidence of drug allergy is usually lower than what parents relate, labeling a patient allergic to drugs (especially the beta lactams) may lead to use of alternate, less effective, more toxic, more broad spectrum, and commonly more expensive drugs. Hence, a proper diagnosis of drug allergy is most important.
In true drug allergies, typical symptoms, such as itching, sneezing, and wheezing, will be present. Urticaria and angioedema are seen frequently. Classic anaphylaxis is rare but is obviously the most serious manifestation.
Symptoms of true drug allergy may include the following:
Pruritus
Pruritic rashes, such as urticaria
Abdominal cramping and diarrhea (as an isolated symptom, probably not due to allergy)
Wheezing
Symptoms of anaphylaxis (described elsewhere in this chapter)
Common nonallergic symptoms that many parents falsely perceive to be allergic include the following:
A nonitchy maculopapular exanthem, especially with amoxicillin
Loose bowel movements, seen with many antibiotics
Stomach upset or vomiting, seen with erythromycin
Diagnostic Criteria
The provider can narrow down the possibility of drug allergy by reviewing a detailed and careful history. If suspicion for true allergy is low, such as a nonpruritic, nonurticarial rash after amoxicillin, then he or she can give an oral challenge in the office and observe the patient carefully for an hour. (The office should be equipped and personnel trained to handle anaphylaxis.)
In situations in which suspicion is high and the suspected drug is indeed the treatment of choice, then the provider should consider consultation with an allergist. He or she should consider skin testing for drug allergy in these patients. Skin tests, however, are hindered by the fact that, for most drugs, appropriate antigens are not available. Even for penicillin allergy, all necessary testing reagents (especially the minor determinants of penicillin allergy) are not yet commercially available, making the task of proper diagnosis difficult. Physicians experienced with these procedures, such as allergists, should perform skin testing and graded oral challenges.
Providers can use the following steps when diagnosing drug allergy:
A detailed history
Skin testing if available, such as with penicillin
RAST testing if skin testing is not possible
Oral drug challenge
Patch testing in cases in which the route of entry is contact through skin in drugs used topically
Management
Acute reactions are treated based on symptoms. Mild pruritic reactions are best treated with antihistamines. Bronchodilators may be necessary for wheezing. Anaphylaxis will require adrenaline, as discussed in the module on anaphylaxis. Once the diagnosis is established, avoidance is advised.
Clinicians commonly face the problem of the development of true allergic symptoms, such as hives, in the middle of a course of treatment. Unless the underlying illness is life threatening, the clinician should not initiate another antibiotic for at least 72 hours, because ongoing hives to the first prescription may be confused for an allergic reaction to the second.
Common drug allergy problems encountered by pro-viders are discussed below.
Penicillins
In case of penicillin allergy, if an alternate drug is not available, then providers should initiate oral or parenteral desensitization.
• Clinical Pearl
Nonpruritic, macular papular rashes seen with amoxicillin are common. They are not IgE mediated and do not represent penicillin allergy. If the rash is urticarial and pruritic, then the provider should suspect allergy.
Newer studies suggest that the incidence of reactions to a cephalosporin is no greater in patients with true penicillin allergy than for the general population (Matloff, 1999). This contradicts the conclusions of older studies, in which cross-reactivity was felt to be as high as 10% to 15%. This new information has led to oral cephalosporins being used routinely, albeit cautiously, in penicillin-allergic patients. Recent studies have concluded that most children will “outgrow” penicillin allergy (Mendelson, 1998).
Cephalosporins
The molecular structures of second- and third-generation cephalosporins are sufficiently distinct so that when a patient has an allergic reaction to one member of the class an
alternative cephalosporin with a different side chain will probably be well tolerated. The serum sickness-like reactions seen with cefaclor are extremely common and have led to a marked decrease in its use. They are non-IgE mediated, and the provider can safely prescribe another cephalosporin.
alternative cephalosporin with a different side chain will probably be well tolerated. The serum sickness-like reactions seen with cefaclor are extremely common and have led to a marked decrease in its use. They are non-IgE mediated, and the provider can safely prescribe another cephalosporin.
Sulfonamides
Reactions may vary from mild rashes to severe life-threatening eruptions, such as Stevens-Johnson syndrome. For this reason, any reaction to a drug in this class should lead to prompt discontinuation and avoidance of future use of any sulfa drug.
Aspirin and Nonsteroidal Anti-inflammatory Drugs
Aspirin and NSAID reactions are not IgE mediated but nonetheless can be life threatening. Avoidance of all aspirin and NSAIDs is advised. Acetaminophen would be the alternative drug of choice. The patient may tolerate trisalicylate and salsalate.
Radiocontrast Agents
Radiocontrast agent reactions, though involving histamine and other mediators released from mast cells, are not IgE mediated but can lead to life-threatening reactions. Prophylactic treatment consists of using low osmolar nonionic radiocontrast agents. Pretreatment with antihistamines and steroids may prevent reactions from occurring.
REFERENCES
Chips, B. E., Valentine, M. D., Kagey-Sabotka, A., Schuberth, K. C., & Lichtenstein, L. M. (1980). Diagnosis and treatment of anaphylactic reactions to hymenoptera stings in children. Journal of Pediatrics, 97, 177–184.
Kwiterovich, K. A., Kagey-Sobotka, A., Valentine, M. D., & Lichtenstein, D., Golden, B. K., et al. (1997). Natural history of Hymenoptera venom sensitivity in adults. The Journal of Allergy and Clinical Immunology, 100, 760–766.
Ebner, C. (1998). Immunological mechanisms operative in allergen specific immunotherapy: The diagnosis and management of anaphylaxis. The Journal of Allergy and Clinical Immunology, 101 (6 Part 2).
Fasano, M. B., Wood, R. A., Cook, S. A., & Samoson, H. A. (1992). Egg hypersensitivity and adverse reactions to measles mumps and rubella vaccine. Journal of Pediatrics, 120, 878–881.
Fireman, P., & Slavin, G. Atlas of allergies. (2nd ed.).
Golden, D. B. K., Kwiterovich, K. A., Kagey-Sobotka, A., Valentine, M. D., & Lichtenstein, L. M. (1996). Discontinuing venom immunotherapy: Outcome after 5 years. Journal of Allergy and Clinical Immunology, 97, 579–587.
Hendrik, K., Van Halteren, et al. (1997). Discontinuation of yellow jacket venom immunotherapy: Follow up of 75 patients by means of deliberate sting challenge. The Journal of Allergy and Clinical Immunology, 100, 767–770.
Lang, D. M. (1995). Hazards of beta-blockers. Anaphylactoid and anaphylactic reactions. Drug Safety, 12(5), 299–304.
Marquart, D., & Wasserman, S. (1993). Anaphylaxis. In Middleton et al. (Eds.), Allergy: Principles and practice (pp. 1525–1536). St. Louis: Mosby.
Matloff, S. M. (1999). In R. A. Dershowitz (Ed.). Ambulatory pediatric care. Philadelphia: Lippincott-Raven.
Mendelson, L. M. (1998). Adverse reactions to beta lactam antibiotics. Immunology and Allergy Clinics of North America, 745–757.
Middleton, Reed, & Ellis. (1996). Allergy principles and practice (4th Ed.).
Schwartz, L. B., et al. (1989). Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. Journal of Clinical Investigation, 83(5), 1551–1555.
Valentine, M. D., Schuberth, K. C., Kagey-Sobotka, A., Graft, D. F., et al. (1990). The value of immunotherapy with venom in children with allergy to insect stings. New England Journal of Medicine, 323, 160–163.
Visscher, P., Veller, R., & Camazine, S. (1996). Removing bee stings. Lancet, 348, 301.
PART III Tick-Borne Infections
Sunil K. Sood MD
INTRODUCTION
Tick-borne diseases are common causes of morbidity in children in the United States. An inordinate degree of fear exists in the public, as well as some confusion about prevention and treatment among health care providers. This chapter summarizes current knowledge and provides practical guidelines appropriate for primary care clinicians to implement.
LYME DISEASE
Lyme disease, an infectious disorder caused by the spirochete Borrelia burgdorferi sensu lato, is the most common vector-borne infection in the United States. When the term sensu lato (“in a broad sense”) is used, it includes strains not present in the United States, especially Borrelia afzelii and Borrelia garinii, which cause Lyme disease in Europe. The vectors in North America are black-legged ticks of the genus Ixodes. These ticks are widely distributed throughout the United States, yet only a few areas are considered endemic for Lyme disease. The following states have the highest incidence rates and account for more than 90% of cases: Connecticut, Rhode Island, New York, New Jersey, Pennsylvania, Maryland, Wisconsin, and Minnesota (Dennis, 1998). In the northeastern and north-central regions, high populations of deer and mice sustain transmission of the spirochete, resulting in a higher infection rate in the deer tick variety of Ixodes. Because small mammals, such as the white-footed mouse (Peromyscus leucopus) can bring ticks into parks and around homes and can nest in backyard debris, children can be exposed in the “peridomestic” environment.
The number of cases of Lyme disease is still increasing and underreported. Primary care clinicians must continue to report new cases to local health authorities so that they can maintain accurate statistics. They can use these statistics to plan environmental measures and immunization strategies.
• Clinical Pearl
Most children (89% in a prospective series) present with single or multiple forms of erythema migrans (EM). This finding should greatly help correct the mistaken perception that Lyme disease is unrecognizable until it is too late to be easily treated.
Arthritis (7%), facial palsy (3%), aseptic meningitis (1%), and carditis (0.5%) are less common symptoms (Gerber, Shapiro, Burke, Parcells, & Bell, 1996). The original classification of clinical manifestations into stages I to III has been replaced by early (generally within 8 weeks after the tick bite) and late Lyme disease. Providers should recognize that extracutaneous involvement can manifest within days of the tick bite.
Pathology
Early-Localized and Early-Disseminated Lyme Disease
Erythema Migrans
Early-localized Lyme disease manifests as a single lesion at the site of inoculation called EM. Mild systemic symptoms may accompany the rash: In one study, 24% of children had fever, 33% had arthralgia, 42% complained of headache, and 58% complained of fatigue (Gerber et al., 1996). It is presumed that at this stage of the infection, spirochetal invasion is restricted to the skin in most patients, but dissemination to the central nervous system (CNS) is occasionally subclinical (Kuiper et al., 1994). A recent discovery is that B. burgdorferi strains vary in their ability to disseminate and cause systemic disease depending on their outer surface protein C (OspC) type.
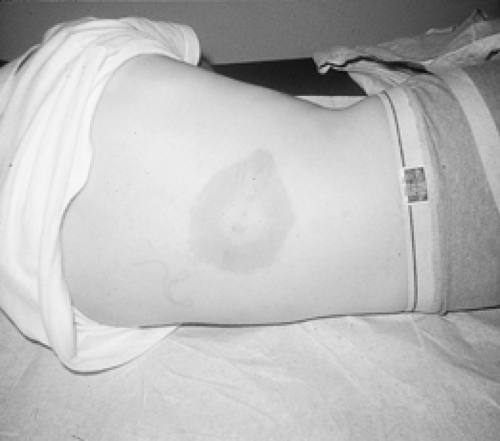
The incubation period after the tick bite is up to 1 month. Typically, EM is somewhat circular and enlarges gradually. It is hardly evanescent, expanding and lasting for 1 to 2 weeks.
• Clinical Pearl
Central clearing, thought previously to be the hallmark of the lesion, is absent in two thirds of cases.
Clearing is a function of the time the rash has been present. Thus, the rash may present as a red plaque, which could be confused with cellulitis. It is painless and nonpruritic, but the patient may experience mild stinging. Figure 48-2 illustrates EM with central clearing.
• Clinical Pearl
An EM diameter of at least 5 cm is an important diagnostic consideration, even in children.
Vesicular, urticarial, scaling, purpuric, and linear-shaped variants occur. A careful examination of the scalp is important because all or part of the rash may occur above the hairline (EM occurs on the head or neck in about 25% of children). As part of the differential diagnosis, smaller ringlike lesions may represent a local allergic reaction to an insect bite, a small area of nummular eczema, tinea corporis, or a reaction to tick saliva that usually fades without expansion. The important consideration is that these lesions do not expand, as does EM.
• Clinical Pearl
A common fear is that Lyme disease will be undetected because not all patients develop the rash; however, 89% of affected individuals do develop the rash either in single or multiple form. Its expanding nature, large size, and continued presence for 1 to 2 weeks leads to early detection in the vast majority of cases in endemic areas (Gerber et al., 1996).
Multiple Erythema Migrans
Multiple EM, a consequence of hematogenous spread of the infection, may be confused with erythema multiforme. The main distinction is that EM lesions are circular and not raised, though varying in size, whereas erythema multiforme consists of lesions of varying morphology, including some urticarial lesions.
Neurologic Infection
Peripheral neuropathy (usually VII nerve palsy) and lymphocytic meningitis are common neurologic manifestations of early-disseminated Lyme disease. Even in an endemic area, most Bell’s palsy is idiopathic, but providers should always obtain serology for Lyme disease. Positive serology is usually an indication for antibiotic treatment, whereas there is no established treatment modality for Bell’s palsy (Jain et al., 1996).
Controversy exists about the necessity to perform a lumbar puncture in every child with facial palsy due to Lyme disease (Sood, 1998). Most experts believe that it is unnecessary in the absence of clinical signs of meningeal irritation or headache. A long-term study of untreated facial palsy in Swedish children, including some with cerebrospinal fluid (CSF) pleocytosis, revealed no long-term sequelae (Niemann et al., 1997). Still, it is important to assess the need for lumbar puncture carefully, because in the presence of meningitis, the treatment of choice will be an IV antibiotic. Lyme meningitis is a lymphocytic meningitis with elevated protein levels. Several cases complicated by pseudotumor cerebri have been reported (Kan, Sood, & Maytal, 1998).
Other cranial neuropathies very rarely are due to Lyme disease, but providers should investigate any unexplained palsy for Lyme disease. Bannwarth’s syndrome of radiculoneuritis, typically nerve pain parasthesia or weakness originating in the cervical or thoracic nerve roots, is uncommon in North American Lyme disease and is rarely encountered in U.S. children. Nevertheless, providers should consider Lyme disease in the differential diagnosis of limb paresthesias, motor or sensory deficits, or neuralgia, especially in the cervical and thoracic dermatomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree