Environmental
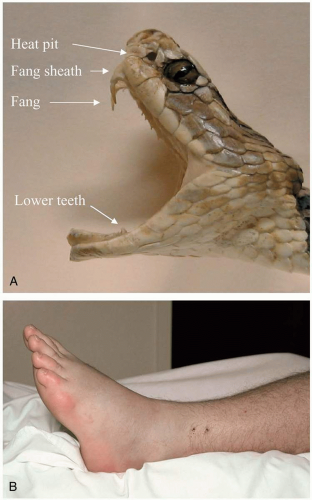
25-1 Snake Bite: North American Crotalid
Anthony Morocco
Clinical Presentation
Dry bites, which occur in up to 20% of cases, result in no significant symptoms. After true envenomation, patients quickly develop pain and edema at the envenomation site. Edema spreads from the bite site, with development of ecchymoses and bullae. Patients may exhibit a variety of systemic signs and symptoms, including anxiety, nausea, vomiting, diarrhea, diaphoresis, dyspnea, confusion, hypotension, tachycardia, and fasciculations.1
Pathophysiology
The Crotalidae family of snakes, known as pit vipers, includes the most important venomous snakes in the United States. These include the genera Crotalus (rattlesnakes such as Eastern and Western diamondback), Sistrurus (Massasauga and pigmy rattlesnakes), and Agkistrodon (copperhead and cottonmouth).1
Crotalid venom contains a multitude of cytotoxic ingredients. Endothelial damage by polypeptides and metalloproteinases causes tissue edema and ecchymoses, and thrombin-like glycoproteins cause production of unstable fibrin clots, leading to hypofibrinogenemia, fibrin degradation, and bleeding. Platelets are consumed at the envenomation site, resulting in thrombocytopenia. The Mojave rattlesnake is unique among crotalids, because its venom contains a neurotoxin that causes neuromuscular blockade.1
Diagnosis
The diagnosis of envenomation is made with an appropriate history and typical physical examination findings. Laboratory tests should include creatine phosphokinase (CPK), creatinine, hemoglobin, platelets, prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, and fibrin degradation products.1
Clinical Complications
Rhabdomyolysis can occur, with subsequent myoglobininduced renal failure. Bites to the face can result in edema and airway compromise. Other complications include hypovolemic shock, anaphylaxis, seizures, and multiorgan system failure. Acute reactions and serum sickness can complicate antivenom administration.1
Management
Immobilization of the affected area and rapid transport to a hospital are the most important field interventions. Devices such as a venom extractor may be of some benefit. A sheep-derived Fab fragment antivenom is available and is indicated for significant progression of signs and symptoms or evidence of coagulopathy. Aggressive supportive care and airway management are indicated. Heparin and blood products are not useful in treating crotalid venom-induced thrombocytopenia and disseminated intravascular coagulation (DIC)-like syndromes. Surgical débridement or fasciotomy is rarely necessary.1
REFERENCES
1. Walter FG, Bilden EF, Gibly RL. Envenomations. Crit Care Clin 1999;15:353-386.
25-2 Snake Bite: North American Coral Snake
Anthony Morocco
Clinical Presentation
Little or no local reaction may be noted, even with significant envenomation, although patients may complain of mild swelling or paresthesias at the bite site. Symptom onset may be delayed by 13 hours or longer. The Eastern and Texas coral snakes produce more significant neurotoxicity than the Arizona species does. Reported symptoms from one series of Eastern coral snake bites included paresthesias (35%), vomiting (25%), weakness (15%), diplopia (10%), dyspnea (10%), diaphoresis (10%), muscle pain (10%), fasciculations (5%) and confusion (5%).1 Cranial nerve dysfunction, including dysarthria and hypersalivation, may also occur.
Pathophysiology
Coral snakes are neurotoxic members of the Elapidae family found in the United States and Mexico; they include the Western (Micruroides euryoxanthus), the Arizona (M. euryoxanthus euryoxanthus), the Eastern (Micrurus fulvius), and the Texas (M. fulvius tenere) coral snakes, as well as several Mexican Micrurus species. The coral snakes are known for their bright coloring, which consists of red, yellow, and black bands. In the United States, the saying, “red on yellow, kill a fellow; red on black, venom lack” differentiates the dangerous coral snakes from harmless varieties.2
 FIGURE 25-2 Western coral snake. North American identification is aided by the mnemonic, “red on yellow, kill a fellow; red on black, venom lack.” (© Dr. Julian White) |
Clinical Complications
Death from respiratory failure within as little as 4 hours after envenomation has been reported. Seizures have also been reported, most commonly in children. Antivenom treatment may result in anaphylaxis, anaphylactic reactions, or serum sickness.
Management
Application of a compression bandage and immobilization of the affected area immediately after a bite may slow the lymphatic spread of the venom. Aggressive supportive care, including airway management, is indicated. All patients with suspected coral snake bites should be admitted and observed for at least 24 hours. An equinederived M. fulvius antivenom is available in the United States and is effective for the Eastern and Texas coral snakes, but not the Arizona coral snake. The initial dose is three to six vials. A Micrurus species antivenom is also produced in Mexico. Antivenom treatment is indicated for any patient with a known coral snake bite. Treatment may be much less effective if delayed until after symptom onset.1
REFERENCES
1. Walter FG, Bilden EF, Gibly RL. Envenomations. Crit Care Clin 1999;15:353-386.
2. Gomez HF, Dart RC. Clinical toxicology of snakebite in North America. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:619-644.
25-3 Snake Bite: Elapids
Anthony Morocco
Clinical Presentation
No significant envenomation occurs in up to 80% of bites by elapids such as the Australian death adder. Elapids such as the taipan and the krait are known for their neurotoxic effects; local pain, edema, and tissue necrosis can be a significant effect of the bites of many species, including cobras. In general, systemic symptoms include nausea, vomiting, abdominal pain, headache, and dizziness. Neurologic manifestations usually begin with ptosis and diplopia, and then dysarthria, with subsequent generalized muscle weakness and respiratory failure.1,2
Pathophysiology
The Elapidae family is a group of snakes found in the Americas, Africa, Asia, and Australia. The Elapids include several of the most toxic snakes known, including all of the highly poisonous terrestrial snakes of Australia and other deadly varieties such as cobras and mambas. A few of the notable genera are Pseudonaja (brown snakes), Pseudechis (black snakes), Acanthophis (death adders), and Oxyuranus (taipans) in Australia; Micrurus (coral snakes) in the Americas; Naja and other cobras and Dendroaspis (mambas) in Africa; and Bungarus (kraits—the cobras, including Ophiophagus hannah, the king cobra) and Calliophis (coral snakes) in Asia.1,2
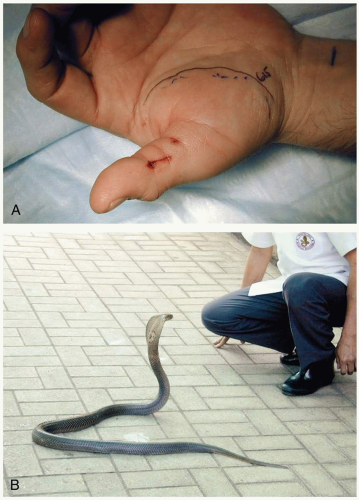 FIGURE 25-3 A: Elapid bite on thumb. (Courtesy of Anthony Morocco, MD.) B: Elapid species include the monocellate cobra, seen here. (Courtesy of Michael Greenberg, MD.) |
Elapid venom varies among species but generally contains substances that result in neurotoxicity, cytotoxicity, and hematotoxicity. Elapids are best known for the neurotoxic components of their venom; these include α-toxins and phospholipases A2, which cause paralysis by acting presynaptically and postsynaptically at the neuromuscular junction. Mamba venom contains unique toxins that block neuronal potassium channels (dendrotoxins) and inhibit cholinesterases (fasciculins).1,2
Diagnosis
Clinical Complications
Bites to the face can result in edema and airway compromise. Unconsciousness and seizures can occur in less than 60 minutes after severe envenomations. Other potential complications include hemolysis, renal failure, compartment syndrome, shock, anaphylaxis, and death. Acute reactions and serum sickness can complicate antivenom administration.1,2
Management
Initial first aid includes immobilization of the affected area and transport to a hospital. Aggressive supportive care and airway management are indicated. Various antivenoms are available, mostly horse-derived and varying by geographical area, that may be effective against one or several species of elapids and members of other snake families.1,2
REFERENCES
1. White J. Clinical toxicology of snakebite in Australia and New Guinea. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:595-617.
2. Warrell DA. Clinical toxicology of snakebite in Asia. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:493-594.
25-4 Snake Bite: Vipers
Anthony Morocco
Clinical Presentation
After envenomation, substantial local effects may develop within minutes to hours. Severe local edema, ecchymoses, bullae formation, tissue necrosis, and regional lymphadenopathy occur. The most severe signs and symptoms occur after Bitis species and Echis species bites. Tissue injury may spread from the bite site to involve an entire limb and part of the torso. Patients may also develop widespread hemorrhage and ecchymoses. Systemic symptoms may include nausea, vomiting, diarrhea, abdominal pain, hyperventilation, dizziness, and chest tightness. A few species can cause mild neurotoxic effects such as ptosis and other cranial nerve abnormalities.1,2
Pathophysiology
The vipers, family Viperidae (or subfamily Viperinae), are a group of medically important snakes found in Europe, Africa, and Asia. A few of the notable species are Vipera berus (adder), Vipera aspis (asp), and Vipera ammodytes (long-nosed viper) in Europe; Bitis species (e.g., Bitis arietans, the puff adder), Echis species (saw-scaled or carpet vipers), and many others in Africa and the Middle East; and Daboia russelii (Russel’s viper) as well as Echis and Vipera species in Asia. These snakes inhabit a wide variety of forest, mountain, and desert areas.1,2
Vipers inject their venom through long, hollow, folding fangs. The venom contains a number of ingredients, including hyaluronidase, histamine, myotoxins, fibrinogenases, kallikrein-like glycoproteins, clotting-factor activators, neurotoxins, and many others. Direct cytotoxicity, platelet destruction, and coagulation-factor consumption occur.1,2
Diagnosis
Laboratory tests should include creatine phosphokinase (CPK), creatinine, hemoglobin, platelets, prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, and fibrin degradation products.
Clinical Complications
Massive third-spacing of fluids and hemorrhage due to coagulopathy can result in shock. Bites to the face can result in edema and airway compromise. Other potential complications include hemolysis, renal failure, compartment syndrome, myocardial injury, pulmonary edema, anaphylaxis, and death. Acute reactions and serum sickness can complicate antivenom administration.1,2
Management
Initial first aid includes immobilization of the affected area and transport to a hospital. Aggressive supportive care and airway management are indicated. Various antivenoms are available, mostly horse-derived and varying by geographical area, that may be effective against one or several species of vipers as well as members of other snake families. Surgical débridement or fasciotomy is rarely necessary.1,2
REFERENCES
1. Warrel DA. Clinical toxicology of snakebite in Africa and the Middle East/Arabian Peninsula. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:433-492.
2. Meier J, Stocker KF. Biology and importance of venomous snakes of medical importance and the composition of snake venoms. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:367-412.
25-5 Snake Bite: Sea Snakes
Anthony Morocco
Clinical Presentation
Patients may present with a dry bite, which results in no symptoms, in 80% of cases. The bite is usually painless, and no local symptoms occur even with significant envenomation. Symptom onset occurs in 30 minutes to 3 hours, starting with myalgias, dizziness, weakness, nausea, vomiting, and dry mouth. The myalgias may progress to severe pain and myolysis. Up to 60 hours after envenomation, patients may develop ptosis, dysarthria, and diminished deep tendon reflexes.1
Pathophysiology
Sea snakes are all members of the family Hydrophiidae; they are found near shores or reefs in the tropical waters of the western Pacific and Indian Oceans. Sea snakes are not aggressive, and most bites are sustained by traditional fisherman who inadvertently capture or step on a snake.1
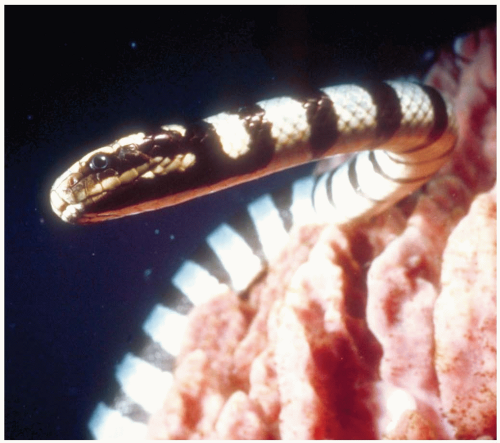 FIGURE 25-5 Sea snakes contain potent venoms. Any case involving systemic envenomation by a sea snake requires treatment with antivenom. (©Robert Yin, 2004. Used with permission.) |
The venom is injected through small, hollow fangs. Unlike most land snakes, the sea snake often holds its bite onto the victim for a prolonged period. The venom contains myotoxins and neurotoxins. The myotoxins are similar to those of crotalid species and cause hyaline necrosis. The neurotoxins bind to the postsynaptic acetylcholine receptor at the neuromuscular junction. Hematotoxicity does not occur.1
Clinical Complications
The combination of vomiting and glossopharyngeal muscle impairment may result in aspiration of gastric contents. Myoglobinuric renal failure occurs due to rhabdomyolysis. Deaths may occur from respiratory muscle paralysis.1
Management
Application of a compression bandage and immobilization of the affected area immediately after a bite may slow the lymphatic spread of the venom. Aggressive supportive care, including airway management, is indicated. Creatine kinase (CK), urine myoglobin, blood urea nitrogen (BUN), and creatinine concentrations should be monitored while administering intravenous fluids to maintain good urine output. Sea snake antivenom is indicated if systemic signs and symptoms of envenomation are present.1
REFERENCES
1. White J. Clinical toxicology of sea snakebites. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:159-170.
25-6 Scorpion Envenomation
Anthony Morocco
Clinical Presentation
Initial symptoms include local pain, pruritus, and hyperesthesia. Mild local erythema and edema may be evident. Systemic signs and symptoms arise within 5 to 30 minutes and may include restlessness, photophobia, dysphagia, diaphoresis, vomiting, diarrhea, dyspnea, cough, lacrimation, salivation, bronchorrhea, muscle spasms and fasciculations, and hypertension or hypotension.2 After Centruroides exilicauda envenomation, tapping on the envenomation site may elicit pain, even in the absence of other local findings. Young children may exhibit agitation, flailing, and roving eye movements.1,2
Pathophysiology
The Scorpiones class of arachnids are all venomous, but fewer than 50 species are considered to be medically important. These include Tityus (Central and South America and the West Indies), Centruroides (southwestern United States, Central and South America), Leiurus (Middle East), Buthus (North Africa, Middle East, southern France and Spain), Androctonus (North Africa and Middle East), Bothotus (India), and Parabuthus (southern Africa). These creatures typically are nocturnal, and they are often encountered hiding in dark areas such as shoes or piles of blankets or clothing. Centruroides exilicauda (bark scorpion) is the most important species in the United States.1,2
 FIGURE 25-6 The scorpion in the jar stung this man’s thumb, causing intense pain and pruritus. (Courtesy of Robert Hendrickson, MD.) |
The scorpion’s tail ends in the bulbous telson, which contains the venom gland and a sharp stinger called the aculeus. Scorpion venoms differ among species but generally contain peptides that affect neuronal and muscle sodium, potassium, and ryanodine-sensitive calcium channels. The venoms also cause release of catecholamines.1,2
Clinical Complications
Severe or fatal effects such as respiratory depression or arrest, shock, pulmonary edema, heart failure, seizures, and coma may occur. Children and the elderly are more severely affected. Other reported complications include pancreatitis, hyperglycemia, allergic reactions, and spontaneous abortion. Serum sickness can occur after antivenom therapy.2
Management
Although antivenoms are available for several species of scorpion, most patients have mild symptoms that can be managed with application of ice and administration of analgesics. Aggressive supportive care and antivenom administration are indicated for severe envenomations. Benzodiazepines may be useful for agitated patients.2
REFERENCES
1. Walter FG, Bilden EF, Gibly RL. Envenomations. Crit Care Clin 1999;15:353-386.
2. Gibly R, Williams M, Walter F, McNally J, Conroy C, Berg RA. Continuous intravenous midazolam infusion for Centruroides exilicauda scorpion envenomation. Ann Emerg Med 1999;34:620-625.
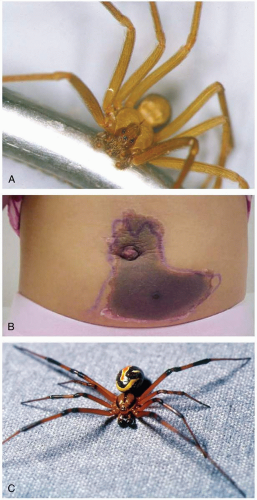
25-7 Widow Spider Envenomation
Anthony Morocco
Clinical Presentation
The initial bite usually causes little or no pain. After 5 to 60 minutes, more severe pain occurs, which may spread to include the regional lymph nodes. Local edema, warmth, and sweating may occur. The local symptoms may be brief and give way to systemic effects (latrodectism) up to 12 or more hours later, particularly after Latrodectus mactans envenomation. Generalized, severe, cramping pain may occur, with abdominal symptoms mimicking those of an acute abdomen. Regional diaphoresis may occur at or distant to the bite site. Hypertension is a common finding; other common symptoms include nausea, vomiting, diarrhea, weakness, psychosis, and headache. The term “facies latrodectismica” refers to the facial grimace, trismus, periorbital edema, and diaphoresis associated with L. mactans envenomation.1,2
Pathophysiology
The widow spiders, of the genus Latrodectus, cause a clinical syndrome known as latrodectism. These spiders can be found worldwide. Five species are found in the United States, including the black widow spider (Latrodectus mactans). Other important species include the brown widow (Latrodectus geometricus) and the redback (Latrodectus hasseltii). The spiders are often found along metal fence lines and in outhouse toilets, sheds, and crop fields. Species such as the black widow are known for their distinctive red hourglass mark on the ventral abdomen.1,2
Diagnosis
Clinical Complications
Management
Treatment of mild to moderate symptoms consists of local ice packs and administration of nonsteroidal antiinflammatory drugs (NSAIDs) or opioids for pain control. Benzodiazepines are helpful to control the painful muscle spasms and to decrease anxiety. Symptoms usually resolve in 24 to 48 hours but may be more prolonged in severe cases. Treatments with calcium and methocarbamol were advocated in the past but are no longer recommended. Horse serum antivenom is available and is highly efficacious in resolving symptoms of severe latrodectism. It is recommended in cases of severe hypertension, intractable pain, or pregnancy. As with any horse-derived antivenom, severe allergic reactions are possible. The antivenom is made using L. mactans, but is likely to be useful for all Latrodectus species.1,2
REFERENCES
1. Clark RF. The safety and efficacy of antivenin Latrodectus mactans. J Toxicol Clin Toxicol 2001;39:125-127.
2. White J, Cardoso JL, Fan HW. Clinical toxicology of spider bites. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:259-329.
25-8 Necrotic Arachnidism
Anthony Morocco
Clinical Presentation
The brown recluse bite is often painless, with development of vesicles, bullae, and erythema after 1 to 3 hours. Pruritus and pain begin as the wound becomes hemorrhagic and erythematous, and the “red, white, and blue sign” appears. The erythema may be wider on the dependent side of the wound, because the venom is pulled inferiorly by gravity. At 24 to 72 hours, the wound becomes pale, edematous, and well demarcated. At this stage, systemic symptoms, which include nausea, vomiting, malaise, and myalgias, may occur. Over the next several weeks, the wound may enlarge, with necrosis and eschar formation. Several other spider species can cause a similar progression of symptoms. The hobo spider can cause a severe headache lasting up to 1 week, with reports of associated hallucinations and paresthesias. The bite of the jumping spider is sharply painful.1
Pathophysiology
The term necrotic arachnidism refers to wounds resulting from envenomation by various spiders. The brown recluse (Loxosceles reclusa) in the southeastern United States, the hobo spider (Tegenaria agrestis) in the northwestern United States, the jumping spider (Phiddipus audax) in the southern United States, and other species have been implicated.1
Loxosceles venom contains a phospholipase known as sphingomyelinase D. This substance causes activation of neutrophils and platelets, with resulting tissue injury, necrosis, and thrombosis.1
Diagnosis
Accurate diagnosis may be difficult, and wounds from diverse causes are often wrongly attributed to the brown recluse. There is no readily available test to confirm the diagnosis.1
Clinical Complications
Ulcers can take months to heal. Severe systemic reactions, including hemolysis, disseminated intravascular coagulation (DIC), and death, may rarely occur after brown recluse envenomation.1
Management
Treatment includes rest, ice, and elevation of the envenomated area. Antibiotic prophylaxis may be helpful for ulcerated lesions.1 Steroids, hyperbaric oxygen, and dapsone have not been shown to be beneficial after Loxosceles envenomation.1 Surgical excision, if considered, should be delayed for 6 to 10 weeks, until the eschar has stabilized.1
REFERENCES
1. Sams HH, Dunnick CA, Smith ML, King LE. Necrotic arachnidism. J Am Acad Dermatol 2001;44:561-573.
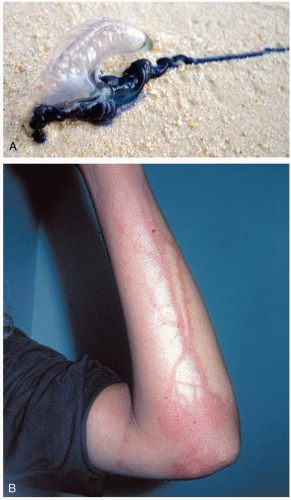
28-9 Portuguese Man-of-War Envenomation
Anthony Morocco
Clinical Presentation
Patients complain of immediate intense pain after envenomation. Elliptical wheals form, with papules and surrounding erythema along the area of tentacle contact. Systemic effects such as nausea, vomiting, headache, myalgias, and respiratory and cardiac compromise may be seen.1
Pathophysiology
The Physalia species are of the phylum Coelenterata (Cnidaria), but they are not true jellyfish of the class Scyphozoa. Instead, they are members of the Hydrozoa class. These stinging creatures consist of a colony of symbiotic organisms that are specialized to perform various tasks, such as reproduction and digestion. The organisms form a blue, air-filled float called the pneumophore, with dangling tentacles up to several feet in length. The two common species are Physalia utriculus, called the “bluebottle,” which is found in the Pacific Ocean, and the larger Physalia physalis, called the “Portuguese man-of-war,” which is found in the Atlantic Ocean. The latter common name refers to the pneumophore, which is similar in appearance to old Portuguese war helmets. The Physalia are often found in large swarms that may wash ashore during the heavy onshore winds of strong tropical storms.1
The precise toxic components of Physalia venom are unclear. It appears to contain dermonecrotic, myotoxic, hemolytic, and cardiotoxic components, as well as vasoactive amines. The venom is delivered by nematocysts, which are cells that contain a harpoon and syringe-like stinging apparatus. The cells discharge after being triggered by mechanical or chemical stimulation. Thousands of nematocysts may be present on one tentacle.1
Diagnosis
Patients may give a history of contact with the easily recognizable blue Physalia. However, the patient may not have seen the pneumophore, because stinging can occur after contact with the end of a long tentacle or fragments of tentacle suspended in the water. In these cases, diagnosis is based on the patient’s clinical presentation and history of potential exposure. There are no specific diagnostic tests.1
Clinical Complications
P. physalis (but probably not P. utriculus) is associated with severe envenomation and systemic toxicity. Deaths due to cardiovascular collapse and respiratory failure have been reported.1
Management
There is conflicting evidence regarding the effect of acetic acid on any undischarged Physalia nematocysts, so this common treatment should probably be avoided. Any remaining nematocysts should be rinsed off with seawater. Local pain should be treated with opioids and ice packs. Supportive care alone is indicated for severe reactions, because no antivenom is available.1
REFERENCES
1. Williamson J, Burnett J. Clinical toxicology of marine coelenterate injuries. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton, FL: CRC Press, 1995:89-115.
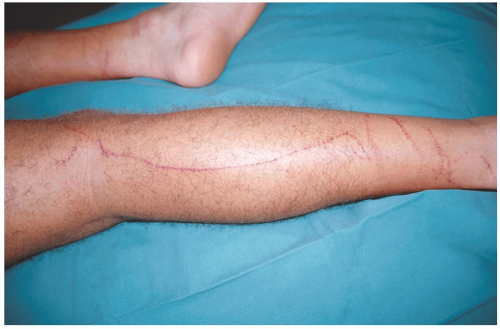
25-10 Box Jellyfish Envenomation
Anthony Morocco
Clinical Presentation
Patients develop a spectrum of effects after envenomation by C. fleckeri, ranging from mild local effects to rapid cardiovascular collapse and death. Severe local pain, linear tentacle marks, vesiculation, and edema, followed by dermatonecrosis, may occur. Fragments of tentacle may be seen on the skin.1,2
Pathophysiology
The box jellyfish, Chironex fleckeri, is found in the waters of northern Australia, with higher numbers present in the summer months. The stinging tentacles may reach 60 m in length and can be fatal to humans. This organism has often been called “the world’s most venomous animal.”1,2
The precise toxic components of C. fleckeri venom are unclear. It appears to contain dermonecrotic, myotoxic, hemolytic, and cardiotoxic components. The venom is delivered by nematocysts, which are cells that contain a harpoon and syringe-like stinging apparatus. The cells discharge after being triggered by mechanical or chemical stimulation. Thousands of nematocysts may be present on one tentacle.1,2
Diagnosis
Clinical Complications
Sixty-seven deaths have been reported after C. fleckeri envenomation. In addition to rapid cardiovascular collapse, hypotension, autonomic dysfunction, and vasospasm may occur. However, despite the reputation for lethality, most stings result in mild symptoms. A delayed hypersensitivity reaction occurs at the site of envenomation in 50% of cases.1,2
Management
Initial first aid should include application of acetic acid to prevent the firing of any remaining nematocysts. Pressure-immobilization bandaging has been advocated but is not likely to be beneficial, and may result in additional nematocyst discharge. In severe cases, patients may require rapid cardiopulmonary resuscitation (CPR). Local pain should be treated with opioids and ice packs. A sheep-derived antivenom is available. Indications for antivenom administration include severe pain unrelieved by opioids, cardiorespiratory decompensation, and cardiac arrest.1,2
REFERENCES
1. Bailey PM, Little M, Jelinek GA, Wilce JA. Jellyfish envenoming syndromes: unknown toxic mechanisms and unproven therapies. Med J Aust 2003;178:34-37.
2. O’Reilly GM, Isbister GK, Lawrie PM, Treston GT, Currie BJ. Prospective study of jellyfish stings from tropical Australia, including the major box jellyfish Chironex fleckeri. Med J Aust 2001;175:652-655.
25-11 Blue-Ringed Octopus Envenomation
Anthony Morocco
Clinical Presentation
The initial bite causes minor bleeding and little or no pain. A significant number of bites are “dry” and produce no further symptoms. Envenomated victims develop perioral paresthesia or numbness that spreads to the tongue and face, followed by diffuse muscle weakness. Difficulty with speech, swallowing, and breathing may occur.1,2
Pathophysiology
The blue-ringed octopus is the only octopus capable of causing significant human toxicity. It is found in the waters of Australia and the Indo-Pacific, with Hapalochlaena maculosa in the south and Hapalochlaena lunulata in the north. The characteristic blue rings appear when the animal is distressed. Most bites occur when an octopus is removed from the water and handled.1,2
 FIGURE 25-11 The blue-ringed octopus (Hapalochlaena spp.) can inject a neurotoxin, which the animal stores in modified venom glands. (©Robert Yin, 2004. Used with permission.) |
Tetrodotoxin, a toxin found in many marine organisms and amphibians, is responsible for the clinical symptoms. It is produced in the salivary glands and is introduced into the victim during a bite from the animal’s beak. The toxin binds to and blocks voltage-gated sodium channels, blocking the inward sodium current responsible for nerve conduction.1,2
Diagnosis
Clinical Complications
Management
There is no specific antidote for tetrodotoxin. Treatment consists of supportive care, including mechanical ventilation, until the effects of the toxin subside over a few hours to several days. Paralyzed patients require sedation, because they may be fully conscious. Acetylcholinesterase inhibitors, such as neostigmine, have been advocated to reverse the paralysis but are of unproven benefit. Patients who are asymptomatic after a suspected bite should be observed for any signs of toxin effects for at least 6 hours.1,2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree