Snakebite causes most mortality attributed to envenomation, particularly in developing countries. The main snake species belong to families of Elapidae and Viperidae. Snake venoms cause neurotoxic, myotoxic, procoagulopathic and anticoagulopathic, cytotoxic, and hemolytic effects.
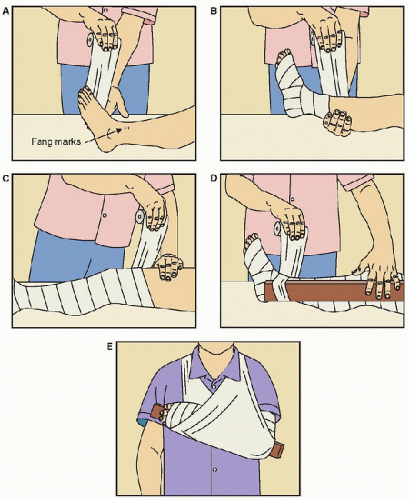
Snakebite causes most mortality attributed to envenomation, particularly in developing countries. The main snake species belong to families of Elapidae and Viperidae. Snake venoms cause neurotoxic, myotoxic, procoagulopathic and anticoagulopathic, cytotoxic, and hemolytic effects. Snakebite is not always accompanied by envenomation, but its management includes first aid that consists of pressure-immobilization bandaging of the bitten limb (for neurotoxic elapid bites), resuscitation, appropriate antivenom therapy, and treatment of systemic effects of venom. Hypoxemia, hypotension, rhabdomyolysis, and disseminated intravascular coagulation may combine to cause renal failure, for which support may be needed. Antivenom administration should be preceded by the administration of subcutaneous adrenaline to prevent and ameliorate adverse reactions.
Snakebite is not always accompanied by envenomation, but its management includes first aid that consists of pressure-immobilization bandaging of the bitten limb (for neurotoxic elapid bites), resuscitation, appropriate antivenom therapy, and treatment of systemic effects of venom. Hypoxemia, hypotension, rhabdomyolysis, and disseminated intravascular coagulation may combine to cause renal failure, for which support may be needed. Antivenom administration should be preceded by the administration of subcutaneous adrenaline to prevent and ameliorate adverse reactions. Bites by spiders are very common, but only a few species of spider threaten life. These include bites by Australian funnel-web spiders; they cause muscle fasciculation followed by weakness and respiratory failure, which along with bronchorrhea is due to acetylcholine release. Hypertension and coma occur secondary to catecholamine release, which later culminates in heart failure, hypotension, and pulmonary edema. An antivenom is available and pressure-immobilization bandaging of the bitten limb is an effective first-aid technique.
Bites by spiders are very common, but only a few species of spider threaten life. These include bites by Australian funnel-web spiders; they cause muscle fasciculation followed by weakness and respiratory failure, which along with bronchorrhea is due to acetylcholine release. Hypertension and coma occur secondary to catecholamine release, which later culminates in heart failure, hypotension, and pulmonary edema. An antivenom is available and pressure-immobilization bandaging of the bitten limb is an effective first-aid technique. Globally, bites by comb-footed spiders, such as the Australian redback spider and the North American widow spider (genus Latrodectus), are the most important. They cause severe pain, inflammatory signs, and hypertension. Antivenoms are available in several countries.
Globally, bites by comb-footed spiders, such as the Australian redback spider and the North American widow spider (genus Latrodectus), are the most important. They cause severe pain, inflammatory signs, and hypertension. Antivenoms are available in several countries. Bites by some North and South American recluse spiders may cause dermatonecrotic lesions alone or in combination with gastrointestinal illness, coagulopathy, hemolysis, and sometimes rhabdomyolysis, with subsequent renal failure. Antivenoms are sometimes available.
Bites by some North and South American recluse spiders may cause dermatonecrotic lesions alone or in combination with gastrointestinal illness, coagulopathy, hemolysis, and sometimes rhabdomyolysis, with subsequent renal failure. Antivenoms are sometimes available. Scorpion stings may threaten life in India, Africa, Brazil, Mexico, and the southern American States. All cause severe pain and, depending on the species, life-threatening cardiovascular effects and a wide range of neurotoxicity. The efficacy of antivenoms is debated, but judicious vasodilator and inotropic therapy, along with mechanical ventilation, may be required.
Scorpion stings may threaten life in India, Africa, Brazil, Mexico, and the southern American States. All cause severe pain and, depending on the species, life-threatening cardiovascular effects and a wide range of neurotoxicity. The efficacy of antivenoms is debated, but judicious vasodilator and inotropic therapy, along with mechanical ventilation, may be required. Bee and wasp stings worldwide and some ant stings may cause life-threatening anaphylaxis. Envenomation syndromes may occasionally be encountered with multiple stings. Prominent bee species are the common honeybee (Apis mellifera) and wasps of the genera Vespa, Vespula, Provespa, and Polistes. Prominent ants are the Australian Myrmecia species (jumping jack and bull-ants) and the American Solenopsis spp. (fire ants). Treatment is as for anaphylaxis and, longer term, venom immunotherapy.
Bee and wasp stings worldwide and some ant stings may cause life-threatening anaphylaxis. Envenomation syndromes may occasionally be encountered with multiple stings. Prominent bee species are the common honeybee (Apis mellifera) and wasps of the genera Vespa, Vespula, Provespa, and Polistes. Prominent ants are the Australian Myrmecia species (jumping jack and bull-ants) and the American Solenopsis spp. (fire ants). Treatment is as for anaphylaxis and, longer term, venom immunotherapy. Tick bite may cause allergy and zoonosis, especially rickettsial diseases, Lyme disease, viral encephalitis, and hemorrhagic fever. Species that cause paralysis in North America are of the genera Dermacentor, Amblyomma, and Ixodes; in South Africa, Argas; and in Australia, Ixodes. The onset of flaccid paralysis is slow. Mechanical ventilation may be required until spontaneous recovery after tick removal.
Tick bite may cause allergy and zoonosis, especially rickettsial diseases, Lyme disease, viral encephalitis, and hemorrhagic fever. Species that cause paralysis in North America are of the genera Dermacentor, Amblyomma, and Ixodes; in South Africa, Argas; and in Australia, Ixodes. The onset of flaccid paralysis is slow. Mechanical ventilation may be required until spontaneous recovery after tick removal. Although many species of jellyfish cause painful stings, few threaten life. Most deaths from jellyfish stings have been due to the chirodropid (jellyfish with many tentacles arising from corners of a bell) Australian box jellyfish (Chironex fleckeri), whose stings cause rapid cardiorespiratory failure by unknown mechanisms. An antivenom is available.
Although many species of jellyfish cause painful stings, few threaten life. Most deaths from jellyfish stings have been due to the chirodropid (jellyfish with many tentacles arising from corners of a bell) Australian box jellyfish (Chironex fleckeri), whose stings cause rapid cardiorespiratory failure by unknown mechanisms. An antivenom is available. Similar chirodropid jellyfish inhabit the Indo-Pacific region. Of the carybdeid jellyfish (single tentacles arising from corners of a bell), the Hawaiian box jellyfish (Carybdea alata), Australian jimble (Carybdea rastoni), and irukandji (Carukia barnesi) cause significant pain, and stings by the latter may be accompanied by hypertension and late cardiac failure. Treatment includes pain relief, oxygen therapy, diuretics, vasodilators, inotropic support, mechanical ventilation or application of continuous positive airway pressure, and possible antihypertensive therapy.
Similar chirodropid jellyfish inhabit the Indo-Pacific region. Of the carybdeid jellyfish (single tentacles arising from corners of a bell), the Hawaiian box jellyfish (Carybdea alata), Australian jimble (Carybdea rastoni), and irukandji (Carukia barnesi) cause significant pain, and stings by the latter may be accompanied by hypertension and late cardiac failure. Treatment includes pain relief, oxygen therapy, diuretics, vasodilators, inotropic support, mechanical ventilation or application of continuous positive airway pressure, and possible antihypertensive therapy. Although stings by Physalia spp. (Portuguese man-of-war, bluebottle) are the most frequent worldwide, few deaths have been recorded.
Although stings by Physalia spp. (Portuguese man-of-war, bluebottle) are the most frequent worldwide, few deaths have been recorded. Numerous fish have spines that inject venom when they are handled or trodden upon, causing very painful lesions. The most prominent are species of the genus Synanceia (stonefish), which inhabit the Indo-Pacific region. An antivenom is available. Occasionally, the barbs of stingrays cause severe penetrating chest trauma.
Numerous fish have spines that inject venom when they are handled or trodden upon, causing very painful lesions. The most prominent are species of the genus Synanceia (stonefish), which inhabit the Indo-Pacific region. An antivenom is available. Occasionally, the barbs of stingrays cause severe penetrating chest trauma. Several species of Australian Hapalochlaena (blue-ringed) octopuses inject TTX, which causes the rapid onset of flaccid paralysis, necessitating mechanical ventilation for several hours.
Several species of Australian Hapalochlaena (blue-ringed) octopuses inject TTX, which causes the rapid onset of flaccid paralysis, necessitating mechanical ventilation for several hours. Some mollusc cone snails fire a venom-laden mini-harpoon, which bears protein toxins that cause rapid paralysis.
Some mollusc cone snails fire a venom-laden mini-harpoon, which bears protein toxins that cause rapid paralysis. Some fish are poisonous to eat, including numerous worldwide tetrodotoxic species (order tetraodontiformes) that cause paralysis. Tropical and subtropical species cause ciguatera, an acute gastrointestinal illness that is accompanied by a polymorphic long-lasting neurologic illness for which mannitol may be effective. Scombroid poisoning is due to consumption of fish that are contaminated by bacteria that manufacture histamine, which causes flushing, tachycardia, hypotension, and bronchospasm. Consumption of some shellfish may cause toxin-related paralysis or gastrointestinal illness.
Some fish are poisonous to eat, including numerous worldwide tetrodotoxic species (order tetraodontiformes) that cause paralysis. Tropical and subtropical species cause ciguatera, an acute gastrointestinal illness that is accompanied by a polymorphic long-lasting neurologic illness for which mannitol may be effective. Scombroid poisoning is due to consumption of fish that are contaminated by bacteria that manufacture histamine, which causes flushing, tachycardia, hypotension, and bronchospasm. Consumption of some shellfish may cause toxin-related paralysis or gastrointestinal illness.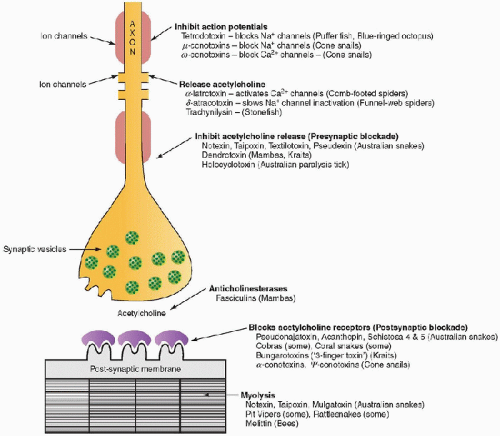 FIGURE 37.1. Sites of action of some major toxins and poisons on nerve, neuromuscular junction, and muscle. |
 Medically significant venomous snakes can be classified into two major families—the Elapidae and Viperidae. Elapids are front fanged terrestrial snakes, and they include most dangerous Australian snakes (taipan, brown, death adder, tiger, and black snakes), the cobras, mambas, and kraits of Asia and Africa, as well as the coral snakes of the Americas. Two coral snakes of medical importance exist in the United States—the eastern coral snake and the Texas coral snake. Elapid venoms are highly neurotoxic with an additional cytotoxicity in some species such as spitting cobras. Vipers have characteristic large front folding fangs and their venom is less likely to cause systemic toxicity than that of the elapids. The venom of vipers is notable for inducing bite site swelling and tissue destruction. These snakes include the rattlesnakes of the Americas, and the
Medically significant venomous snakes can be classified into two major families—the Elapidae and Viperidae. Elapids are front fanged terrestrial snakes, and they include most dangerous Australian snakes (taipan, brown, death adder, tiger, and black snakes), the cobras, mambas, and kraits of Asia and Africa, as well as the coral snakes of the Americas. Two coral snakes of medical importance exist in the United States—the eastern coral snake and the Texas coral snake. Elapid venoms are highly neurotoxic with an additional cytotoxicity in some species such as spitting cobras. Vipers have characteristic large front folding fangs and their venom is less likely to cause systemic toxicity than that of the elapids. The venom of vipers is notable for inducing bite site swelling and tissue destruction. These snakes include the rattlesnakes of the Americas, and the old and new world vipers. A small number of venomous Colubridae, a family of back fanged snakes, are also medically important, such as the African Boomslang. The Hydrophiidae is a fourth family of venomous snakes and includes the sea snakes found along much of the Indo-Pacific coastline, predominantly in the tropics.
Puncture marks (usually on limbs) which:
may be difficult to see;
may consist of single, double, or multiple puncture marks or scratch marks;
may be accompanied by bruising/bleeding/oozing/blistering;
may be multiple, suggesting severe envenomation (although this may occur with nonvenomous snakebites).
Regional tender lymphadenopathy (this, also may be present after bites by nonvenomous snakes, and is thus not by itself an indication for antivenom).
Swelling, bruising/bleeding/oozing/blistering, pain.
Headache, nausea, vomiting, abdominal pain
Collapse, unconsciousness (may be transient).
Painful, tender muscles (myolysis)
Blurred vision, diplopia, difficulty swallowing or breathing, slurred speech, weakness, paresthesia (neurotoxicity)
Spontaneous bleeding from mucosal surfaces, continual bleeding from the bite site or venepunctures (coagulopathy).
Progressive limb swelling, blistering, and discoloration
Irritability, confusion, coma
Bleeding from bite, venepuncture, or other sites (care should be taken with puncture of arterial or central venous sites in the presence of potential coagulopathy, and intramuscular injections should be avoided)
Dark urine (myoglobinuria, hematuria)
Ptosis, dysarthria, weakness/paralysis, dyspnea, respiratory failure (neurotoxicity).
Venom detection at bite site, in urine, or in blood using snake venom detection kit (SVDK) (available only for use in Australia and Papua New Guinea)
Blood tests to include:
Coagulation studies INR/PT, APTT, ACT, D-dimer, X-FDP, and fibrinogen. If these tests are unavailable, perform a test “20-minute whole blood clotting test.” A blood sample in a plain glass tube should clot within 10 minutes, if it remains unclotted at 20 minutes, coagulopathy is present.
Creatine kinase level to check for myolysis.
Renal function tests to determine if renal function is impaired secondary to myoglobinuria or hypotension.
Electrolytes because rhabdomyolysis may cause elevated K+ and decreased Ca++ levels.
Complete blood count, while the white cell count is usually acutely mildly elevated, significant leucocytosis may indicate other pathology. Thrombocytopenia may occur with some snakebites in isolation, as part of disseminated intravascular coagulation (DIC), or due to microangiopathic hemolytic anemia.
Urinalysis to check for hemoglobin, myoglobin.
ECG and cardiac troponin in cases of suspected cardiotoxicity. This may be primary or secondary, for example in the case of hyperkalemia associated with rhabdomyolysis.
nonvenomous snakebite;
bite or sting by other venomous creature (e.g., Hymenoptera, spider, octopus, jellyfish);
cerebrovascular accident;
ascending neuropathy (Guillain-Barré syndrome);
acute myocardial infarction;
allergic reaction (allergy may also develop to snake venoms, as well as to antivenoms);
hypoglycemia/hyperglycemia;
drug overdose;
closed head injury;
sepsis.
is particularly severe in cases of Russell’s viper envenomation. Some snake venoms, including many elapid species, contain both postsynaptic and presynaptic neurotoxins, the latter being difficult to reverse if the patient is not treated promptly. Coagulation disturbances, secondary to consumption procoagulopathy, fibrinolysis, or anticoagulation, are common after many elapid and viper bites, although severe hemorrhage is infrequent. Most Australian snake envenomations can be diagnosed within 12 hours of bite by disturbances of coagulation or neurological disturbances (5).
TABLE 37.1 THE CLINICAL FEATURES OF VARIOUS MEDICALLY SIGNIFICANT VENOMOUS SNAKES OF THE WORLD | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
adverse reaction varies considerably in frequency and severity. Overall, adverse reactions are common and may be divided into early hypersensitivity reactions (true anaphylactic reactions are probably less common compared with anaphylactoid reactions), pyrogenic reactions, and late allergic reactions (serum sickness). Limited data are available to estimate the incidence of each type of reaction. In general, the highest rates of acute reaction, up to 70%-80%, occur in unfractionated equine antivenoms, whereas the incidence of immediate hypersensitivity and serum sickness after ovine North American Crotaline polyvalent immune Fab antivenom is 8% and 13%, respectively (31).