and Melanie Hoehn2
(1)
Division of Vascular Trauma, R Adams Cowley Shock Trauma Center, University of Maryland Medical Center, Baltimore, MD, USA
(2)
University of Maryland Medical Center, Baltimore, MD, USA
Keywords
Endovascular therapyNational Trauma Data Bank (NTDB)SheathsCathetersGuidewiresThoracic endovascular aortic repair (TEVAR)Stent graftSeat belt aortaAbdominal aortic injuryMinimal aortic injuriesDissections of the aortaPseudoaneurysmsResuscitative endovascular balloon occlusion of the aorta (REBOA)Intravascular ultrasound (IVUS)Introduction
The use of interventional procedures in trauma has increased steadily over the past 10 years. An analysis of the National Trauma Data Bank (NTDB) reported that 8.1 % of acute arterial injuries in 2003 were treated with endovascular therapy, compared to only 2.1 % in 1994. Nearly an equal number of blunt (55 %) and penetrating (45 %) injuries were treated with endovascular therapy [1]. A more recent study, also using NTDB data, reported that 16 % of traumatic vascular injuries were treated with endovascular therapy, including 20 % who were hypotensive at the time of intervention. The authors report decreased mortality with the increased use of endovascular intervention [2]. With advancements in both imaging and device technology, the use of endovascular techniques has become part of the treatment algorithm for the injury.
Endovascular therapy in trauma involves a minimally invasive, catheter-based approach. Sheaths, catheters, and guidewires are universal instruments, regardless of procedure. Devices passed over guidewires form the basis of diagnosis and treatment.
Thoracic Aortic Injury
History of Care
Prior to the endovascular era, care of thoracic aortic injury had been open repair. Early aortic stent grafting was based on treatment of thoracic aortic disease. These devices were not tailored to the younger, disease-free aorta of most trauma patients. As a result, many device-related complications occurred in the early stages of the endovascular era.
Assessment and Operative Technique
Immediate assessment of the patient with suspected blunt thoracic aortic injury (BTAI) includes imaging studies if hemodynamics allow. Once the diagnosis is confirmed, aggressive beta-blockade is highly recommended in the absence of contraindications. The decision regarding endovascular versus open repair is made largely on the position of the lesion relative to arch vessels. Traditionally, a proximal landing zone of 2 cm was considered acceptable, although newer devices have been used successfully with a proximal neck length of 1.5 cm. Aortic injuries are usually shorter than lesions due to aortic disease, negating the need for a preoperative spinal drain.
Injuries to the thoracic aorta that are within proximity to the major branch vessels present a challenge when the proximal or distal landing zones for the device include the take-off of major branch vessels. Coverage of the left subclavian artery (LSCA) has been routinely performed with minimal morbidity. Close observation is usually sufficient; in the event that symptoms develop and progress, a carotid-subclavian bypass is required. Other situations, such as prior cardiac surgery, may necessitate a bypass procedure prior to stent-graft placement. Careful preoperative assessment of vertebral artery patency, dominance, and flow direction must be performed in the event that the LSCA origin needs to be covered. Intraoperative neuromonitoring can provide immediate information regarding cerebral flow after coverage of the LSCA. Cerebral angiography may also be performed if absolutely needed.
Bilateral femoral artery cutdown or percutaneous cannulation is obtained. Access sheaths for wires, catheters, and sheaths are advanced under fluoroscopy. Pre-procedural planning can significantly decrease the amount of contrast used, particularly in patients with concomitant injuries. If uncertainties regarding the lesion exist with angiography, the intravascular ultrasound (IVUS) is used to more accurately assess the injury, as well as to confirm the proximal landing zone and stent apposition and rule out endoleak.
Outcomes
Thoracic endovascular aortic repair (TEVAR) is currently the most studied endovascular intervention in trauma. The short-term data suggests that aortic stent grafting is comparable to open repair, citing lower rates of paraplegia, blood loss, and complications and shorter hospital stays [3–5]. While many have embraced its use, it suffers from a lack of long-term data. The relationship of the aging stent graft to the aging aorta is unknown.
Complications
Complications of TEVAR include stent collapse and “bird beaking” (the proximal portion of the stent graft does not oppose to the aortic wall) due to size mismatching and/or a small arch radius and may ultimately require additional endovascular or open repair. The American Association for the Surgery of Trauma (AAST) multi-institutional trials have documented the complication rates of TEVAR [3]. Many industry-sponsored multi-institutional trials are currently investigating devices with improved deployment systems and conformability, particularly suitable for small arch radius aortas seen in younger patients. Ongoing trials for branched and fenestrated grafts (Fig. 19.1) will allow devices suitable for coverage across arch vessels to be available if proven safe and effective.
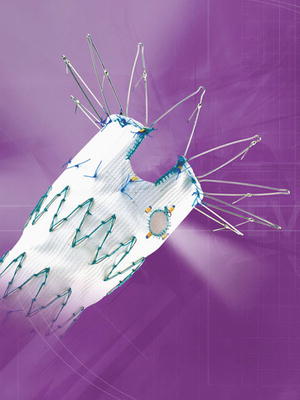

Fig. 19.1
Fenestrated aortic stent graft. For use only in approved trials in the USA.
Abdominal Aorta
Introduction
Abdominal aortic injury (BAAI) occurs from the biomechanical direct and indirect forces applied to the aorta while it is tethered between the spinal column, the peritoneum, and the abdominal viscera. The most frequent force causing abdominal aorta injury is compressive, associated with deceleration [6]. Indirect forces occur when the aortic wall is directly stretched and compressed against a high-pressure column of blood leading to intimal tears, pseudoaneurysm, rupture, or thrombosis. Atherosclerotic changes of the aorta have been associated with a weakening of the intima in addition to the loss of elasticity and compliance and thus thought to increase the risk of aortic injury [7, 8]. These forces can occur during motor vehicle collisions when the aorta is compressed by the seat belt thus termed “seat belt aorta” [6]. Other mechanisms include long-distance falls from heights, direct compression of the aorta, and penetrating injuries.
The underlying pathologic lesion of BAAI is the disruption of the intima. Depending on the magnitude of force, BAAI presents as a spectrum of disease ranging from a minimal aortic injury (MAI) to free rupture of the aorta. With increasing use of computed tomography angiography (CTA) for diagnosis of BAAI, there have been increasing numbers of MAI diagnosed [9]. Traumatic intimal tears can occur and manifest as intimal flaps or dissection. Furthermore, these can be complicated by thrombosis [10] and acute arterial insufficiency [11–13]. Injuries involving the adventitia lead to pseudoaneurysm formation (Fig. 19.2) or even rupture of the aortic wall. Rupture of the aortic wall can also be due to branch vessel avulsion.
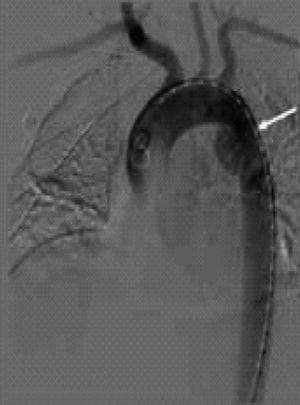

Fig. 19.2
Traumatic aortic pseudoaneurysm.
The majority of BAAI occur inferior to the renal arteries. They are classified based on the presence of aortic contour abnormality and presence of free rupture [14]. This classification is based on experience with BTAI [15]. Intimal tears/flaps are not associated with external aortic contour abnormality. A pseudoaneurysm presents as a contained rupture and is clearly associated with an aortic contour abnormality. The most common associated injuries are small bowel injuries (38 %) and thoracolumbar spine injuries (25 %). The most common reported BAAI are intimal tears/flaps (41 %) followed by pseudoaneurysms (29 %) [14].
Assessment
The initial presentation is dependent on the presence or absence of free rupture of the abdominal aorta, branch vessel avulsion, or concomitant inferior vena cava injury [16]. Those typically present with hemorrhagic shock. In a recent series, all such patients were hemodynamically unstable, and 75 % had cardiac arrest in the emergency department with 38 % receiving an ED thoracotomy [14].
Initial Management
Management of aortic injury varies and risk of mortality rises with the severity of the pathologic lesion. In a recent review of the NTDB of 436 patients with BAAI from 180 centers between 2007 and 2009, 90 % were managed nonoperatively. Of those who underwent operative repair, 69 % underwent endovascular repair, with the remainder undergoing open aortic repair and two extra-anatomic bypasses [18]. In general, cases of BAAI with uncomplicated intimal flaps are managed nonoperatively with beta-blockers and antiplatelet (aspirin) therapy and close follow-up. The natural history in these cases is a decrease in size and complete resolution [19]. Cases of aortic pseudoaneurysm require operative repair to prevent rupture, but this can be managed on a semi-elective basis during the initial hospitalization. Cases complicated by thrombosis, acute arterial insufficiency, and free ruptures require repair. The timing of repair also depends on associated injuries. Patients with pulse and neurologic deficits, an expanding hematoma, and concomitant intra-abdominal injuries require emergent repair. Otherwise, treatment can be individualized in the hemodynamically stable multisystem trauma patient.
Operative Techniques
Endovascular stent placement for BAAI is an attractive alternative for patients with both isolated BAAI and multisystem trauma in a manner similar to that of BTAI (Fig. 19.3). Most of the literature is limited to case reports and small case series where endovascular stents, aortic extension cuffs, and limb extensions have all been used. Indications include cases with associated gross contamination from hollow viscus injury that can jeopardize aortic grafts due to risk of infection or management of injuries difficult to expose by open exploration. Endovascular interventions can be used as a stabilizing measure for critically ill patients and a bridge to open definitive repair.
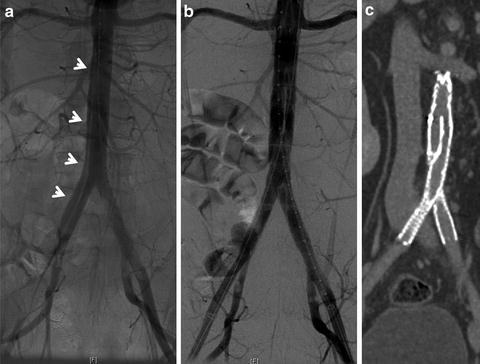

Fig. 19.3
Infrarenal blunt abdominal aortic dissection. (a) An intraoperative arteriogram showing the dissection flap (arrows) in the infrarenal aorta and extending to the right common iliac artery. (b) Arteriogram post-endovascular stent graft placement. (c) Coronal view of CT angiography 6 weeks post repair.
Abdominal aorta zones of injury can be classified [14] based on feasibility of endovascular approach as follows: Zone I injuries from diaphragmatic hiatus to the superior mesenteric artery (SMA). Zone II injuries are between the SMA and renal arteries. Zone III injuries are inferior to the renal arteries to the aortic bifurcation. Zone I and III injuries are amenable to endovascular repair, whereas Zone II lesions are not without fenestration for the SMA and renal arteries. The timing of the repair is dependent on the patient’s hemodynamic status and the presence of acute limb ischemia.
In the abdominal aorta, major injuries near the visceral and renal vessels are not amenable to stent placement. Technologic advancements such as fenestrated (Fig. 19.1) or branched grafts are only used in the USA in trials for elective aortic surgery, but have been used with success in other countries. They may be available in the future for off-label use in the injured aorta. Additionally, the chimney technique, in which the branch vessels are stented along with the aorta, may be an option for select patients. Unfortunately, this technique is time consuming and requires a high degree of experience. Rarely, an aortic injury occurs where the proximal and distal landing zones do not place branch vessels at risk, and a stent is an excellent and minimally invasive treatment option (Fig. 19.4) [20].
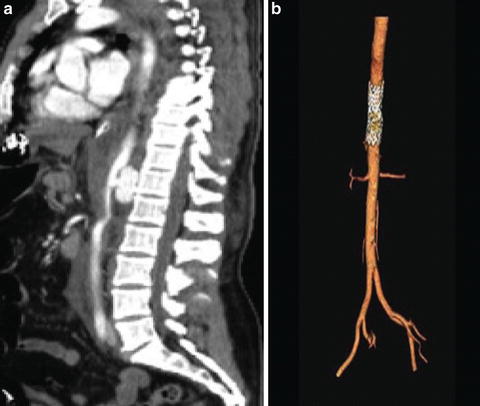

Fig. 19.4
Distal thoracic aortic injury (a) repaired with aortic stent graft seen on postoperative CTA with reconstruction (b).
Outcomes
BAAI is highly lethal. Among those who survive the transport to the hospital mortality rates range from 32 to 78 % with hemorrhagic shock being the most common cause of mortality [16, 21, 22]. Outcomes in cases requiring a resuscitative thoracotomy remain poor [23, 24]. In BAAI, mortality varies by the type of aortic injury. When minimal aortic injuries, dissections of the aorta, and pseudoaneurysms are included, aggregate mortality is 11 % [14]. In cases treated by endovascular repair, the long-term durability of aortic endografts for abdominal aortic trauma has not been well described. Clearly long-term follow-up will be required in these cases. The ongoing AAST multi-institutional PROOVIT and AORTA trials will provide some answers in the near future.
Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA)
History
The most common cause of death from aortic trauma remains hemorrhagic shock compounded by ongoing coagulopathy; thus, early proximal control of the aorta is a life-saving maneuver [14, 16, 21, 22]. The intra-aortic occlusive balloon placed through an open approach was first described for controlling major aortic hemorrhage in the Korean War [25]. Reports of use for control of bleeding during pelvic surgery [26, 27], hepatobiliary surgery [28], orthopedic surgery [29], postpartum hemorrhage [30], and repair of ruptured AAA [31, 32] suggest that resuscitative endovascular balloon occlusion of the aorta (REBOA) is a life-saving measure. Physiologic parameters such as serum lactate, pH, pCO2, and central, cerebral, and coronary perfusion in animal models of hemorrhagic shock have been shown to improve with the REBOA [33–36]. Descriptions of its use for trauma are few [14, 37–39], but indications include control of noncompressible torso hemorrhage in the abdomen and pelvis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








