Endocrine Emergencies
KEY POINTS
1 Severe hypothyroidism and hyperthyroidism must be suspected and treated on clinical grounds; laboratory tests are often delayed too long to be useful. When beginning treatment for hypothyroidism, consideration must be given to concomitant adrenal insufficiency.
2 Endocrine disorders often have subtle presentations in the ICU. Altered mental status (diabetic ketoacidosis, hypothyroidism, and hyperthyroidism), failure to wean from mechanical ventilation (adrenal insufficiency and hypothyroidism), and refractory hypotension (adrenal insufficiency, diabetes insipidus) are the most common clinical presentations in which endocrinologic causes are overlooked.
3 Maintain a high suspicion of adrenal insufficiency. When suspected, diagnostic investigation (adrenocorticotrophic harmone stimulation test) and institution of therapy (isotonic saline and corticosteroids) should be undertaken simultaneously.
4 To avoid hypoglycemia make every effort not to interrupt regular feedings; avoid oral hypoglycemics and long-acting insulin; and check bedside blood glucose values frequently.
5 Fluid administration, low-dose insulin, and potassium replacement are mainstays in the care of diabetic ketoacidosis. Reduction in the anion gap is the single most informative laboratory test.
6 Because hypertonic glucose is responsible for maintaining the circulating volume in patients with hyperosmolar non-ketotic coma, adequate volume resuscitation is essential before beginning insulin therapy.
7 Suspect diabetes insipidus when large volumes of dilute urine are accompanied by hyperosmolar hypernatremia. In such patients, it is probably most prudent to confirm the diagnosis by supplementing antidiuretic rather than performing a water deprivation test, which can prove fatal in the critically ill patient.
▪ THYROID DISEASE
Critical Illness and Thyroid Testing
Mild hyperthyroidism and hypothyroidism are very common in the ambulatory population, but severe disease in the intensive care unit (ICU) is rare. Nevertheless, profound excess or deficiency of thyroid hormone is life threatening, can be confused with many other nonendocrine conditions, and is generally amenable to simple therapy. By far the most common “thyroid disorders” seen in the ICU are not thyroid problems at all but rather laboratory anomalies resulting from altered binding and metabolism of thyroid hormone caused by critical illness or drug therapy. Critical illness has numerous effects on thyroid tests, in some cases simulating disease when none is present and in other cases obfuscating a true diagnosis. For example, almost all critical illnesses decrease thyroid-stimulating hormone (TSH) and the plasma concentration of proteins that bind thyroid hormone (albumin, thyroid binding prealbumin, thyroxin binding globulin). As binding proteins decrease, total levels of thyroxin (T4) and to a lesser degree triiodothyronine (T3) decline, simulating hypothyroidism. In fact more than 50% of critically ill patients have subnormal T4 and T3 levels. Historically, the term “euthyroid sic” was used to describe the constellation of low T4, T3, and TSH but it now appears perhaps some of these patients actually have transient central hypothyroidism. Nevertheless, when T4 or T3 is administered to critically ill patients with these laboratory findings clinical outcomes are not improved. Critical-illness-induced falls in plasma protein concentrations can also obscure a true diagnosis of hyperthyroidism by lowering observed T4 and T3 levels into the normal or near normal range. (Most patients with hyperthyroidism have an increased T3, even if T4 levels are normal.)
Drugs commonly used in the ICU also complicate thyroid function test interpretation by inhibiting TSH secretion, T4 binding to serum proteins, or T4 to T3 conversion. For example, glucocorticoids,
octreotide, dobutamine, dopamine, and dopamine agonists inhibit TSH secretion, potentially leading to an erroneous diagnosis of central hypothyroidism if T4 values are low or to an erroneous diagnosis of hyperthyroidism if T4 is modestly elevated. Highly protein-bound drugs like phenytoin, carbamazepine, furosemide, aspirin, and some nonsteroidal anti-inflammatory drugs displace T4 from binding proteins, lowering total T4 levels, and potentially leading to a false diagnosis of hypothyroidism. Finally, glucocorticoids, amiodarone, and β-blockers all inhibit the peripheral conversion of T4 to T3, resulting in a low serum T3 concentration and encouraging an incorrect diagnosis of hypothyroidism.
octreotide, dobutamine, dopamine, and dopamine agonists inhibit TSH secretion, potentially leading to an erroneous diagnosis of central hypothyroidism if T4 values are low or to an erroneous diagnosis of hyperthyroidism if T4 is modestly elevated. Highly protein-bound drugs like phenytoin, carbamazepine, furosemide, aspirin, and some nonsteroidal anti-inflammatory drugs displace T4 from binding proteins, lowering total T4 levels, and potentially leading to a false diagnosis of hypothyroidism. Finally, glucocorticoids, amiodarone, and β-blockers all inhibit the peripheral conversion of T4 to T3, resulting in a low serum T3 concentration and encouraging an incorrect diagnosis of hypothyroidism.
Given the many influences of critical illness on thyroid function tests, several guiding principles should be kept in mind: (a) Do not send “screening” thyroid function studies; tests should be thoughtfully selected to confirm or exclude a specific suspected condition. (b) Unlike ambulatory practice, where clinical examination and a TSH measurement usually suffice to diagnose both hypothyroidism and hyperthyroidism, use of TSH alone in the ICU for diagnosis is fraught with problems. (c) Be suspect of thyroid disease diagnoses made in critically ill patients.
Severe Hyperthyroidism and Thyroid Storm
No absolute signs differentiate the flowery term “thyroid storm” from severe hyperthyroidism, although diagnostic criteria have been proposed. Apart from accentuated signs and symptoms of hyperthyroidism, thyroid storm is more likely to exhibit fever (often approaching 103°F) and tachycardia (pulse > 100/min). Secondary features may include a goiter, proptosis, congestive heart failure, arrhythmias, tremor, diaphoresis, diarrhea, elevated liver function tests, and mental status changes.
Historically, surgery performed on large goiters with poor preoperative preparation was the most common cause of thyroid storm, but currently, the condition most often results from an acute infection, withdrawal of antithyroid drugs, or nonthyroid surgery. Recognizable Graves disease is present in most patients with severe hyperthyroidism. (Toxic multinodular goiter and autonomous thyroid nodules are also fairly common causes.) Although iodine ingestion initially increases T4 production, it suppresses T4 release. However, serum iodine levels decline after 10 to 14 days, allowing discharge of large amounts of newly formed T4 into the circulation. For this reason, radioactive iodine and IV and oral iodinated radiographic contrast may precipitate delayed thyroid storm in predisposed individuals. Accidental or intentional overdose with exogenous T3 or T4 is only problematic following massive ingestions.
In the critically ill patient with suspected thyroid storm, the diagnosis must initially be a clinical one. The results of thyroid function tests are often delayed and comparable elevations in total T4, free T4, and T3, may occur in both mild and severe hyperthyroidism (Table 32-1). When normal or only modestly elevated T4 or T3 levels are seen in patients with clinically overt hyperthyroidism, the finding is usually due to reductions in binding protein levels induced by critical illnesses. TSH should be undetectable (<0.01 mU/L) in essentially all cases of true hyperthyroidism, whereas low but detectable levels of TSH (0.01 to 0.1 mU/L) associated with normal or modestly elevated T3 and T4 levels are usually the result of critical illness alone. Among patients with Graves disease, often only T3 concentrations are elevated (T3 thyrotoxicosis).
Elevated hepatic transaminases indicate lifethreatening disease. Rarely, hypercalcemia may be present. In thyroid storm, the leukocyte count is usually normal or slightly elevated, but relative lymphocytosis is common, a feature that may help to differentiate thyroid disease from infectious causes of fever.
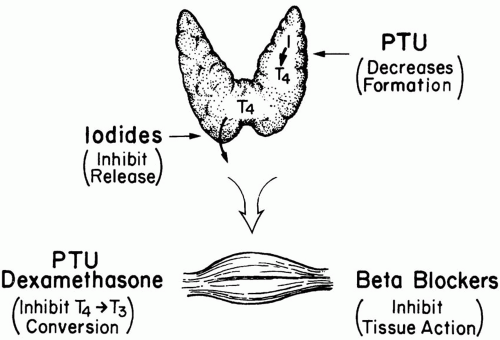
The treatment of thyroid storm is fourfold: (a) block T4 formation, (b) prevent T4 release, (c) inhibit peripheral conversion of T4 to T3, and (d) block the tissue effects of T4 (Fig. 32-1). Propylthiouracil (PTU) and methimazole both block T4 synthesis within hours but do not stop release of preformed
T4 from the thyroid. Of the two choices, PTU (600 mg load followed by 200 mg PO q4-6h) is preferred because it also stops conversion of T4 to its biologically active form (T3) in peripheral tissues. (Inhibition of T4 to T3 conversion may also be accomplished by glucocorticoids and to a lesser degree by propranolol.) Although PTU is preferred over methimazole, allergic reactions may occur with its use, and delayed, dose-dependent agranulocytosis is a risk. Low doses of PTU represent a particularly good choice of therapy for the pregnant patient because the drug crosses the placental barrier poorly. On the other hand, an advantage of methimazole is its long duration of action, allowing less-frequent dosing compared to PTU. If methimazole is chosen, an initial dose of 60 mg followed by 30 mg every 6 h is reasonable.
T4 from the thyroid. Of the two choices, PTU (600 mg load followed by 200 mg PO q4-6h) is preferred because it also stops conversion of T4 to its biologically active form (T3) in peripheral tissues. (Inhibition of T4 to T3 conversion may also be accomplished by glucocorticoids and to a lesser degree by propranolol.) Although PTU is preferred over methimazole, allergic reactions may occur with its use, and delayed, dose-dependent agranulocytosis is a risk. Low doses of PTU represent a particularly good choice of therapy for the pregnant patient because the drug crosses the placental barrier poorly. On the other hand, an advantage of methimazole is its long duration of action, allowing less-frequent dosing compared to PTU. If methimazole is chosen, an initial dose of 60 mg followed by 30 mg every 6 h is reasonable.
TABLE 32-1 LABORATORY ANALYSIS OF THYROID FUNCTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Release of preformed T4 into the circulation is rapidly blocked by a supersaturated oral solution of iodine (five drops [250 mg] of supersaturated potassium iodide [SSKI] solution q6h) or by dexamethasone (2 mg IV q6h). Unfortunately, even complete blockade of T4 release does not terminate hyperthyroid crisis because circulating T4 has a very long half-life, and conversion to T3 continues. Peripheral conversion can be inhibited by corticosteroids or propranolol. Because PTU and iodine are only available as enteral preparations, administration may prove difficult in the critically ill. Because iodine inhibits thyroid uptake of PTU and methimazole, these drugs must be administered at least 2 h before iodine therapy.
β-blockers blunt the tissue actions of thyroid hormone, (especially in the heart) but must be used with caution, particularly in patients with congestive heart failure or bronchospasm. If a β-blocker is used, it makes some sense to initially select a short-acting agent such as esmolol to gauge the patient’s response. If adverse consequences develop, the effects can be rapidly terminated. If tolerated, propranolol and metoprolol are reasonable long-term choices. In deliberate ingestions of thyroid hormone, oral administration of bile acid sequestering drugs can prevent absorption. In severe overdoses, plasmapheresis or peritoneal dialysis may be used to remove thyroid hormone from the circulation.
Heart failure occurs in half of patients with thyroid storm. Although classically described as a “high-output” state, many patients have normal or low cardiac outputs and elevated pulmonary artery occlusion pressures and may be harmed by the use of β-blockers. By contrast, hypertension and tachycardia seen in thyroid storm may respond well to β-blockade. Associated tachyarrhythmias may be controlled with a combination of digoxin, β-blocker, and calcium channel blockers. Fever from increased metabolic rate may be managed by direct external cooling, but aspirin and NSAIDs should be avoided because of their tendency to displace T4 from serum proteins, possibly worsening thyroid storm. Therapy should also include nutritional support because of heightened caloric requirements. (Folate and
B vitamins are also rapidly consumed and should be supplemented.) Increased thyroid activity accelerates the metabolism of many drugs, including some of those useful in its treatment (β-blockers and dexamethasone), thus larger doses on a more frequent schedule may be necessary.
B vitamins are also rapidly consumed and should be supplemented.) Increased thyroid activity accelerates the metabolism of many drugs, including some of those useful in its treatment (β-blockers and dexamethasone), thus larger doses on a more frequent schedule may be necessary.
Severe Hypothyroidism (Myxedema Coma)
Hypothyroidism is a very common condition but its most severe manifestation, “myxedema coma,” is quite rare. It is hypothermia, central nervous system (CNS) dysfunction, bradycardia, and hypotension that differentiate myxedema coma from simple hypothyroidism. Myxedema is an important reversible cause of several common syndromes in the ICU including (a) severe ileus suggestive of bowel obstruction, (b) respiratory failure (including failure to wean from a ventilator), (c) heart failure, (d) hypothermia, and (e) coma. Myxedema coma is a serious disorder, with up to 30% of victims succumbing to the illness and its complications.
In more than 90% of cases, hypothyroidism results from primary failure of the thyroid gland, not pituitary insufficiency. Making a de novo diagnosis of myxedema in the ICU is uncommon. Most patients hospitalized with severe hypothyroidism have carried the diagnosis for some time; their admission is usually precipitated by the combination of discontinued thyroid replacement therapy and/or intercurrent illness (e.g., infection, myocardial infarction, surgery, hypothermia, trauma, and drugs, particularly sedatives). For unclear reasons, hypothyroidism is much more likely to occur in older patients, in women, and in the winter months. Lithium and amiodarone are rare causes.
The diagnosis of myxedema coma must be a presumptive one because thyroid function tests results may be delayed and therapy should be initiated promptly to optimize outcome. The history is often one of slowly progressive lethargy, depression, and mental dullness. Common physical signs include (a) obesity, (b) dry, puffy skin, (c) characteristic facies, (d) sinus bradycardia, (e) decreased relaxation phase of deep tendon reflexes, and (f) nonpitting edema. A previous thyroidectomy scar is an obvious clue. Pleural and pericardial effusions and ascites are also often detected. Less commonly, hoarseness, macroglossia, and hair loss are noted. Paradoxically, hypertension is as common as hypotension.
Normocytic anemia, hyponatremia, hypoglycemia, hypercapnia, and hypoxemia are frequent but not diagnostic. When hypoglycemia is noted, concurrent adrenal insufficiency is common. Marked elevations in cholesterol are also frequently noted and above normal levels of creatine, phosphokinase may be noted seen. The hyponatremia seen in hypothyroidism is often multifactorial, reflecting inappropriate antidiuretic hormone (ADH) secretion and combined treatment with diuretics and hypotonic fluids.
The laboratory hallmark of primary hypothyroidism is an elevated TSH concentration (usually >20 mU/L) accompanied by low total and free T4 levels (see Table 32-1). (The much less common secondary or “pituitary” form of the disease is characterized by a low T4 and low TSH.) Many nonthyroidal illnesses decrease the T3 and total T4 levels without impairing thyroid function, the so-called sic euthyroid state. In this syndrome, decreased T4 binding protein and altered T4 metabolism lead to a decreased total T4 with normal or slightly depressed free T4, a normal or reduced T3, and normal or minimally elevated TSH. The sic euthyroid condition is not benefitted by thyroid hormone replacement therapy. Rarely, long-term dopamine infusion may inhibit pituitary release of TSH suggesting a sic euthyroid state in a patient with true primary hypothyroidism. Because orally administered drugs are absorbed poorly in myxedema, T4 should initially be administered IV. Controversy exists because no controlled studies have been conducted to guide replacement amounts, but reasonable starting daily doses of T4 range from 0.2 to 0.5 mg IV. (There is no benefit from larger initial doses.) Commonly, 0.5 mg of T4 is given over the first 24 h, followed by 0.1 mg each day thereafter. In normotensive, euthermic patients the initial dose may be as low as 0.1 mg. Clinical improvement usually begins within 12 to 24 h. Without good data it is commonly believed that T4 doses should be reduced in diminutive and elderly patients and those with known coronary disease because abrupt replacement may precipitate ischemia.
Because the peripheral conversion of T4 to T3 in myxedema is impaired, some experts advocate administration of T3 (10 μg) directly. This practice had not been proved to be more effective than giving T4 alone, is much more expensive, and had been associated with an increased risk of death if not carefully monitored.
When hypothyroidism is suspected, adrenal function must also be tested because a hemodynamic crisis may be precipitated if T4 is
administered to patients with concurrent adrenal insufficiency. To avoid missing the diagnosis, the most practical approach is to perform an adrenocorticotropic hormone (ACTH) stimulation test when thyroid function tests are obtained, and begin empiric stress doses of glucocorticoids (hydrocortisone 100 mg IV q8h) while awaiting the results (see Adrenal Insufficiency, following). If baseline cortisol levels return greater than 25 μg /dL or double after ACTH hydrocortisone can be discontinued. Hypoglycemia occurs frequently enough to warrant urgent evaluation in acutely ill patients with altered mental status and suspected hypothyroidism. Arterial blood gases (ABGs) should be analyzed in most patients because suppressed ventilatory drive leading to hypercapnic respiratory failure is common. Hyponatremia is effectively treated by temporary water restriction. Hypotension and hypoperfusion should be treated with T4 and corticosteroids as well as fluids and vasopressors as dictated by usual hemodynamic parameters. Many patients will have concomitant hypothermia that is best treated with passive external rewarming (see Chapter 28).
administered to patients with concurrent adrenal insufficiency. To avoid missing the diagnosis, the most practical approach is to perform an adrenocorticotropic hormone (ACTH) stimulation test when thyroid function tests are obtained, and begin empiric stress doses of glucocorticoids (hydrocortisone 100 mg IV q8h) while awaiting the results (see Adrenal Insufficiency, following). If baseline cortisol levels return greater than 25 μg /dL or double after ACTH hydrocortisone can be discontinued. Hypoglycemia occurs frequently enough to warrant urgent evaluation in acutely ill patients with altered mental status and suspected hypothyroidism. Arterial blood gases (ABGs) should be analyzed in most patients because suppressed ventilatory drive leading to hypercapnic respiratory failure is common. Hyponatremia is effectively treated by temporary water restriction. Hypotension and hypoperfusion should be treated with T4 and corticosteroids as well as fluids and vasopressors as dictated by usual hemodynamic parameters. Many patients will have concomitant hypothermia that is best treated with passive external rewarming (see Chapter 28).
Aspiration pneumonitis (often leading to acute lung injury) is a very common complication in patients with reduced mental status secondary to hypothyroidism. Although rare, bacterial meningitis may be a precipitant of myxedema. Thus, it is probably prudent in most of these patients to obtain cultures of relevant body fluids and administer empiric antibiotics until cultures return negative. The obesity and immobility of the profoundly hypothyroid patient predispose to the formation of atelectasis, decubitus ulcers, and deep venous thrombosis. Prevention of these common complications is covered in detail in Chapter 18.
▪ ADRENAL DISEASES
Classical Adrenal Insufficiency
The physiologic effects of adrenal insufficiency result from deficiencies of cortisol and/or aldosterone. Loss of mineralocorticoid action is responsible for most of the significant manifestations seen in the ICU. Regardless of whether the disease results from adrenal (primary) or pituitary gland (secondary) failure, most patients’ symptoms are mild and well compensated until a second process causes volume depletion (vomiting, diarrhea), or vasodilation (sepsis, drugs, surgery). (Until the supervening illness occurs, loss of aldosterone is compensated for by increased salt and water intake.)
The most common and severe form of the disease, primary adrenal insufficiency, results from direct adrenal gland destruction. Tuberculosis, fungal disease, surgery, infarction, metastatic cancer, autoimmune disease, and hemorrhage are the most frequent causes. Hemorrhagic adrenal insufficiency is seen most commonly in septic or in anticoagulated patients, especially in postcardiopulmonary bypass. Critically ill patients with the acquired immunodeficiency syndrome (AIDS) also have a high frequency of adrenal insufficiency. In patients with AIDS, cytomegalovirus, metastatic neoplasm, and ketoconazole therapy are the most common culprits; yet in many patients, the etiology remains obscure. In primary adrenal insufficiency, pituitary secretion of ACTH increases in an attempt to stimulate the inadequate adrenal glands to maintain normal cortisol levels. Because both mineralocorticoid (aldosterone) and glucocorticoid functions are lost, clinical manifestations are more severe and in important respects differ from the secondary form of the syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree