1. Children with diabetes presenting for surgery should undergo careful preoperative assessment and perioperative planning with an effort to optimize the patient’s condition preoperatively and to minimize the disturbances in glucose homeostasis that can result from the stress of surgery and anesthesia in the perioperative period. For emergency surgery, every effort should be made to correct abnormalities as much as possible prior to induction.
2. It is best to schedule surgeries in diabetic children early in the day and it is critical to understand that even fasting diabetic children frequently need insulin. Blood glucose levels should be monitored frequently in the perioperative period and treated if necessary as both hyper-and hypoglycemia have been shown to have deleterious effects.
3. Congenital hyperinsulinism (CHI) is the most common cause of severe persistent hypoglycemia in infancy and can present with pallor, syncope, tachycardia, diaphoresis, and seizures. Immediate treatment is a glucose load with subsequent medical and surgical management based on the form of CHI.
4. If possible, time should be taken to correct children with hypo- and hyperthyroidism to a euthyroid state preoperatively as both diseases predispose to hemodynamic instability under anesthesia. Hypothyroid patients may have an exaggerated response to sedative drugs, impaired drug metabolism, and delayed emergence while those with hyperthyroidism may have increases in drug metabolism and anesthetic requirements.
5. Parathyroid hormone (PTH) is essential for homeostasis of extracellular calcium. Hypocalcemia has greater anesthetic implications than hypercalcemia and can present as hypotension or electrocardiogram (ECG) changes including QT prolongation.
6. The posterior lobe of the pituitary secretes vasopressin (also referred to as antidiuretic hormone, ADH), which functions to maintain fluid balance. An absence of vasopressin can lead to excretion of large amounts of dilute urine, hypovolemia, and hypernatremia (diabetes insipidus, DI) whereas excess vasopressin can cause the opposite disorder with retention of free water, hypervolemia, and hyponatremia (syndrome of inappropriate antidiuretic hormone, SIADH).
7. Prolonged administration of exogenous glucocorticoids can lead to adrenal insufficiency and suppress the body’s ability to produce glucocorticoids in response to stress. These patients require “stress-dose” steroids during periods of illness or surgical stress and hemodynamic instability may be a sign of inadequate replacement.
8. Patients with pheochromocytoma require careful preoperative preparation including α-blockade and restoration of intravascular volume. β-blockade may be beneficial for heart rate control but only after adequate α-blockade has been achieved.
9. Multiple endocrine neoplasia (MEN) syndromes are a collection of familial endocrinopathies that share findings of adenomatous hyperplasia and malignant tumors in different endocrine glands. It is important to recognize the tumors that characterize each type as patients may present with multiple coexisting pathologies.
10. Carcinoid syndrome refers to carcinoid tumors that may elaborate vasoactive amines and polypeptides. Signs and symptoms include cardiovascular perturbations, valvular heart disease, flushing, bronchoconstriction, and diarrhea. Stress and surgery can precipitate carcinoid crisis and medications to treat these symptoms should be immediately available intraoperatively.
It is remarkable that the endocrine and metabolic systems are able to respond to the incredible demands of the various types of growth phases throughout childhood with predictable and reliable ease. As with all systems in the human body, however, abnormalities can and do occur, both in structure and function. The effects of these abnormalities can impact multiple organ systems, all of importance to the anesthesiologist.
I. Diabetes mellitus
A. The most common type of diabetes in children is type 1 (insulin-dependent), most frequently caused by an immune-mediated destruction of pancreatic β cells. These children have no endogenous insulin production and must be maintained on exogenous insulin.
1. The age of onset varies, but the majority of cases are diagnosed in childhood and present with diabetic ketoacidosis (DKA).
2. The prevalence among U.S. youth is increasing; data reports a prevalence of 1.93 per 1,000 children aged 0 to 19 years in 2009, an increase from 1.48 per 1,000 in 2001 (1).
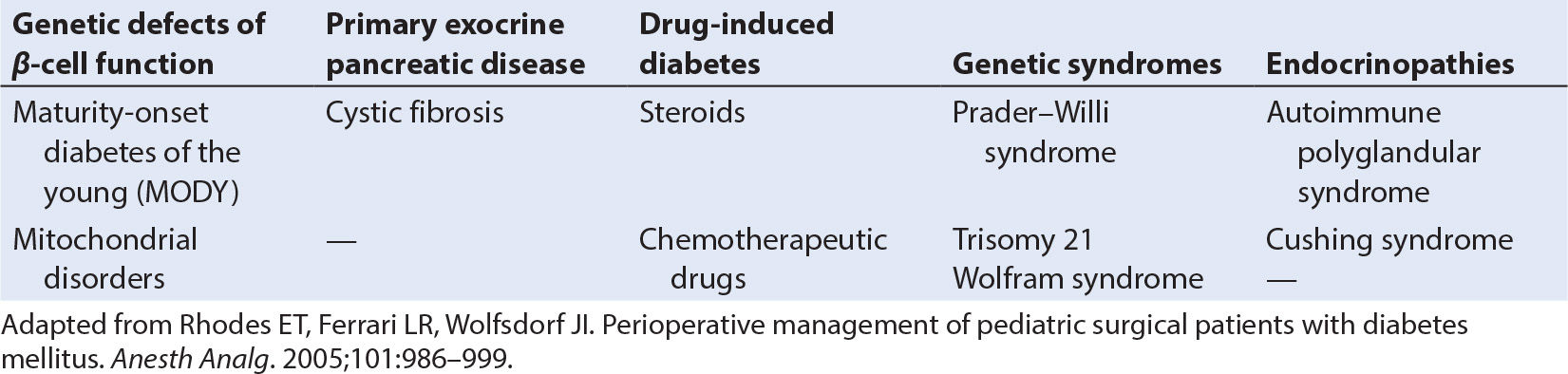
3. In addition to this common form of diabetes mellitus, there are other rare causes of islet cell failure or insulin resistance (Table 29.1).
4. The current management of type 1 diabetes in childhood has undergone significant recent changes as new preparations of insulin and new delivery technologies come into broader use.
5. Euglycemia and the avoidance of long-term complications of diabetes remain the key goal. This is complicated by several factors in childhood.
a. Ongoing growth continually modifies insulin requirements. This is amplified during periods of rapid growth and development such as puberty and adolescence.
b. Behavioral factors make the adherence to strict dietary and activity regimens challenging.
c. The frequency of minor illnesses in childhood, such as upper respiratory infections, may frustrate attempts to maintain glucose homeostasis. Either a pediatric diabetologist or a pediatrician with experience in childhood diabetes will be caring for these children on a long-term basis and their assistance and collaboration should be sought for perioperative management.
6. Maintenance therapy for type 1 diabetes in children is based on the delivery of basal insulin requirements supplemented by intermittent insulin doses to meet the acute metabolic needs of meals and activity.
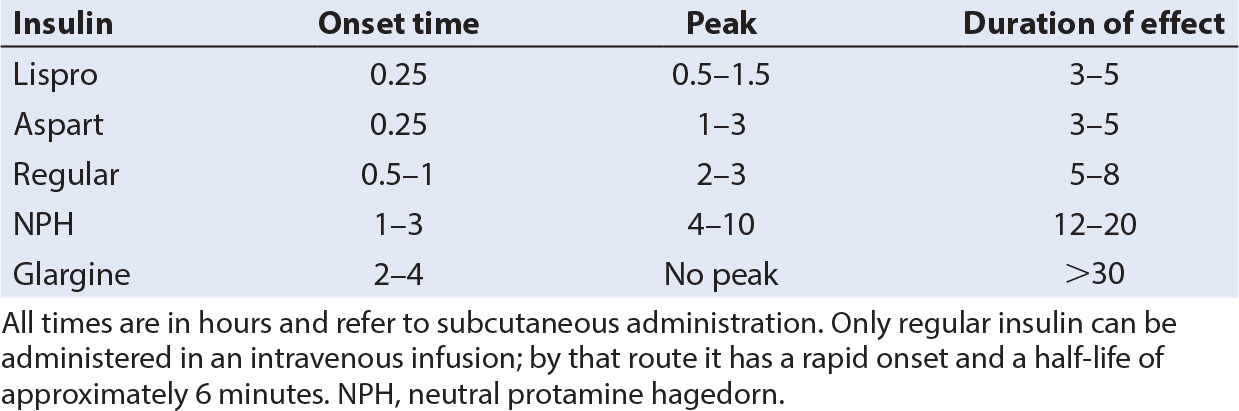
a. While intermediate-acting insulins such as neutral protamine Hagedorn (NPH) or Lente coupled with regular insulin at mealtime have been commonly used in the past, two new maintenance regimens are becoming standard (Table 29.2) (2).
b. Insulin glargine (Lantus, Sanofi-Aventis, Bridgewater, NJ) is a long-acting insulin with no peak, which produces a constant basal level of antiglycemic activity for 24 hours. When used in combination with supplemental doses of rapid-onset/short-acting insulin at meal times, improved glycemic control can be achieved in most children.
c. An alternative approach is to eliminate the use of long- or intermediate-acting insulin entirely, and instead use a continuous subcutaneous infusion of short-acting insulin delivered by a small pump that the child wears at all times. Small frequent boluses to meet the demands of meals can be delivered from the same pump.
7. Both these techniques mandate the close monitoring of blood glucose levels with a portable glycometer using blood samples obtained by frequent finger-sticks and an appropriate response to hyper or hypoglycemia with the goal of maintaining glucose levels in the 100 to 175 range.
a. The advent of new, very rapid-onset, and short-duration insulins, lispro (Humalog, Eli Lilly, Indianapolis, IN) and aspart (Novolog, Novo Nordisk, Princeton, NJ), has made this technique more effective and practical.
b. This is sometimes of benefit in very “brittle” diabetic patients who may require frequent titration of insulin dosing to maintain euglycemia.
B. Type 2 diabetes
1. Although this was rarely reported in children several decades ago, the diagnosis of type 2 diabetes is increasing rapidly in the United States concurrent with the epidemic of childhood obesity (3).
2. In 2009, the prevalence among youth aged 0 to 19 years was 0.46 per 1,000, an increase from 0.34 per 1,000 in 2001 (1). The prevalence varies widely across U.S. racial and ethnic groups.
3. Type 2 diabetes is characterized by insulin resistance and a relative insulin deficiency, but not by pancreatic β-cell failure.
a. Although exercise and dietary management is attempted in these patients, many require the use of some insulin and/or an oral hypoglycemic drug.
b. At this time only metformin is approved for use in children and adolescents.
C. Physiologic responses of the diabetic patient to surgery and anesthesia: Surgery and anesthesia produce stress responses that can precipitate decompensation of even the best-regulated diabetic child.
1. Secretion of stress-related hormones including cortisol, catecholamines, and glucagon inhibits the regulatory effect of insulin and promotes catabolism.
2. Insulin resistance and lipolysis, with the generation of ketones, develop effects similar to those seen during DKA (4).
3. The neuroendocrine responses to tissue injury, including adrenocorticotropic hormone (ACTH) and growth hormone (GH) secretion and a surge in norepinephrine and epinephrine further destabilize the diabetic child.
4. Although preoperative fasting may attenuate the rise in serum glucose that would ordinarily ensue, the catabolic sequelae and their consequences continue unabated.
5. The misconception that there is no need for insulin because the child is fasting has significant negative metabolic consequences. Although opioids and regional anesthesia attenuate some of these stress responses, they cannot ablate them completely. Volatile anesthetics may actually promote some of the same undesirable hormonal effects.
6. The adverse consequences of surgery and anesthesia are multiplied when the diabetic child presents for emergency surgery, where sepsis, electrolyte imbalances, prolonged starvation, and metabolic derangements increase the risk of ketoacidosis and loss of homeostasis. In the postoperative period, fluid and electrolyte imbalance can further deteriorate if close attention is not paid to intraoperative management. Wound healing is impaired in these children and worsened by hyperglycemia and a catabolic state. Wound infections may also be increased due to impaired neutrophil function. It therefore falls to the anesthesiologist to improve outcome by optimizing perioperative management.
D. Preoperative management. Just as with any patient with chronic illness, the goal is to optimize the diabetic patient’s condition prior to an elective procedure. Rhodes et al. have published excellent algorithms to guide the perioperative care of these patients (5).
1. Hemoglobin A1c (glycosylated hemoglobin) levels provide insight into longer-term glucose control and should fall within normal range for age (6% to 8% patients older than 5 years of age, 7% to 9% for those younger than 5 years).
2. The presence of urinary ketones is certainly cause for concern. If reasonable metabolic control is not achieved in the days before elective surgery or if glycemic control is unusually out of range on the day of elective surgery, it may be best to postpone the procedure until better control is achieved.
3. Emergent procedures must usually proceed even in the case of poor glycemic control, but may require postoperative intensive care.
4. Just as in the treatment of DKA, rehydration is critical in these patients and should precede the induction of anesthesia if at all possible, along with at least initial correction of metabolic abnormalities. These patients may have depletion of potassium and phosphate, both of which may have intraoperative hemodynamic consequences and require slow restoration to normal.
5. Metformin should be discontinued the day before any anesthetic as it can precipitate acidosis and elevated lactate should the patient become dehydrated, hypotensive, or hypoxemic.
CLINICAL PEARL For emergent operations in children with diabetes, take time to evaluate and correct metabolic abnormalities as much as possible before induction. Early DKA can get much worse, and is more difficult to correct intraoperatively.
E. Management on the day of surgery will vary depending on the patient’s usual insulin regimen, but there are certain principles that apply in all cases (6).
1. It is typically best to schedule surgeries in diabetic children as the first case of the day in order to minimize fasting times or, if not possible, to place an intravenous cannula early in the day so that fluids, glucose, and insulin can be administered while the child waits for surgery.
2. Blood glucose should be monitored and abnormalities corrected.
3. The use of two separate intravenous infusions (which may be connected to a single cannula), one with and one without dextrose, facilitates the independent titration of volume and substrate.
4. All children with diabetes must have a blood glucose measurement before the induction of anesthesia and periodically during surgery, especially following changes in infusion rates of either insulin or glucose, as intraoperative hypoglycemia may not be easily recognized on clinical grounds and can have serious adverse consequences.
5. The child prescribed glargine for basal glucose control should receive the usual dose on the day of or the evening before surgery.
6. Children on insulin pumps should have their basal infusion rate continued unchanged. Children who are still managed using split-dose insulins (e.g., regular and NPH twice a day) should administer half of their intermediate- or long-acting insulin in the morning only, without any short-acting insulin. If blood glucose levels are low in the morning and the child does not yet have vascular access, a concentrated glucose solution in clear fluid or, for the older child, a glucose-containing lozenge should be offered.
7. Blood glucose levels >250 mg per dL should be treated in the preoperative area.
8. A dose of rapid-acting insulin such as lispro can be administered if the surgery is short and the child is expected to eat after surgery. Rhodes et al. suggest that a correcting dose of lispro that will drop the blood glucose level by 30 mg per dL be calculated by dividing 1,500 by the child’s total daily insulin requirement (in units).
9. Alternatively, an insulin infusion can be started at 0.02 to 0.05 units/kg/hour with a goal of achieving a blood glucose level of 150 mg per dL. This technique is preferable if the surgery is expected to last 2 hours or longer, or if the child will not be able to eat after surgery. Close monitoring of glucose levels is mandatory to titrate the infusion to effect, and a dextrose infusion should be used to correct hypoglycemia or any excessive fall in blood glucose level.
CLINICAL PEARL These patients should have an anesthesia evaluation (even if by telephone) at least a day prior to operation to discuss and implement the preoperative management plan with the parents.
F. Intraoperative management requires the frequent measurement of blood glucose levels. Hypoglycemia must be avoided, and blood glucose target levels of 100 to 150 are sought.
1. If an insulin infusion is used, a glucose infusion should also be provided. The glucose infusion rate can be titrated to achieve blood glucose levels in the target range with the insulin infusion running between 0.02 and 0.05 units/kg/hour.
2. An infusion regimen has been shown to be superior to subcutaneous insulin administration in the perioperative period and should be used for moderate or long-duration surgeries (7).
3. Insulin pumps can either be continued intraoperatively (assuming the anesthesiologist knows how to operate the pump) or the patient can be transitioned to an insulin infusion for the duration of the anesthetic.
4. For very short procedures, such as myringotomy and tubes, an infusion is not necessary, and the child can resume the usual insulin regimen when he/she begins to eat after awakening.
CLINICAL PEARL For operations that do not require arterial monitoring, blood glucose measurements can be obtained by glucometer and “finger stick” if an extremity is accessible, or by placement of a second heparin locked iv.
G. DKA may be a complicating state in the diabetic child who requires emergency surgery. DKA greatly increases the risk of morbidity and mortality and any treatment that can be started before induction is beneficial.
1. These patients are often severely volume-depleted and require fluid resuscitation for their DKA in addition to intraoperative fluid losses. If possible, the initial stages of fluid resuscitation should be completed prior to induction of anesthesia.
2. Because subclinical cerebral edema is a near universal complication of DKA in children, fluid administration must be carefully calculated and titrated to avoid worsening the edema and precipitating brainstem herniation (8).
a. For patients whose dehydration is >10% of body weight, the administration of 4 L per m2 of isotonic fluid over the first 24 hours of resuscitation is advised (9).
b. Insulin can be withheld until the blood glucose level falls below 400, as fluid administration alone will decrease the blood glucose concentration and rapid decreases in blood glucose levels must be avoided.
c. The rate of decrease of blood glucose should not exceed 100 mg/dL/hour.
d. Following assurance of urine output, potassium chloride and phosphate should be added to the resuscitation fluid. Total body potassium depletion despite relatively normal serum levels is typically found in DKA and is a result of intracellular shift.
e. It has been shown that diabetic adults have an impaired cerebral vasodilatory response to hypercapnia, which has obvious adverse implications in the perioperative setting; therefore, alveolar ventilation should be closely monitored (10).
3. Unless the anesthesiologist has experience in the management of DKA in children, the assistance of a pediatric endocrinologist or intensivist should be sought.
II. Hyperinsulinism
A. CHI, although rare, is the most common cause of severe persistent hypoglycemia in infancy (11). Comprised of a group of various genetic disorders, the common finding in CHI is hypoglycemia as a result of unregulated oversecretion of insulin by pancreatic β-cells (12).
1. Symptoms vary based on the severity of hypoglycemia and the age of the individual. Neonates often present with hypoglycemic seizures while infants and children may present with symptoms more commonly associated with hypoglycemia: pallor, syncope, tachycardia, and diaphoresis as well as seizures. Recurrent, severe hypoglycemia can lead to irreversible neurologic damage.
CLINICAL PEARL Hypoglycemia must be considered in the evaluation of seizures in neonates, infants, and children. Failure to diagnose and correct severe hypoglycemia can result in permanent sequelae.
2. Immediate treatment of hypoglycemia secondary to CHI is an enteral or parenteral glucose load. Subsequent medical management to prevent recurrence may consist of glucagon, diazoxide, or octreotide. Surgical options may also be available, depending on the form of CHI.
B. CHI exists in two distinct forms: focal and diffuse.
1. In the focal form, an additional tumor suppressor gene on the short arm of chromosome 11 results in a discrete mass of adenomatous hyperplasia of the islet tissue. The focal type of hyperinsulinemia is amenable to resection of the discrete lesion.
2. The diffuse form is more severe and is caused by mutations that lead to a defective β-cell adenosine triphosphate (ATP)-dependent potassium channel. If medical management fails, subtotal or total pancreatectomy will be necessary, often resulting in insulin-dependent diabetes after surgery.
C. Clinical differences between the two forms are subtle but distinguishing between them is essential to management. Genetics, selective pancreatic arterial calcium stimulation with hepatic vein sampling (ASVS), and positron emission tomography (PET) aid the diagnosis. ASVS and PET are also useful for localizing the lesion in focal CHI.
1. Utilization of ASVS to diagnose CHI and localize lesions requires a multidisciplinary team of endocrinologists, radiologists, surgeons, and anesthesiologists. General anesthesia in the interventional radiology suite is mandatory and the main challenge appears to be the maintenance of stable glucose levels. Deep opioid anesthesia using remifentanil with or without epidural analgesia has been shown to successfully blunt hyperglycemic responses.
a. Different regions of the pancreas are stimulated through selective cannulation of pancreatic arteries, and insulin levels are measured in the venous outflow.
b. A doubling or greater rise in plasma insulin after calcium stimulation localizes the abnormal pancreatic tissue.
c. This technique requires that plasma glucose levels be stabilized between 60 and 90 mg per dL, as fluctuations can generate false positive results.
d. An increased insulin level from all sites is indicative of diffuse disease (13).
2. A newer technique for diagnosis is PET using 18F-fluoro-L-dihydroxyphenylalanine (18F-DOPA), which has a high specificity and sensitivity for detecting lesions producing diffuse or focal hyperinsulinemia (14). This procedure requires up to 2 hours of complete immobility in the computed tomography (CT) suite, and anesthesia is similar to that required by other imaging studies (see Chapter 36). This noninvasive procedure appears to be as accurate as ASVS.
3. If the patient fails medical therapy, subtotal pancreatectomy is required.
a. General anesthesia is induced intravenously with propofol or sodium thiopental and a nondepolarizing neuromuscular blocker is used.
b. An arterial line facilitates frequent blood sampling.
c. Maintenance of anesthesia can be accomplished with a volatile agent and with remifentanil or fentanyl.
d. The use of epidural analgesia is indicated, both for postoperative analgesia and for intraoperative stability as noted earlier.
e. The intraoperative anesthetic management is complicated by continuous changes in blood glucose as resection of pancreatic tissue occurs. Glucose levels should be measured at least every 30 minutes in order to quantify the changes as they occur and adjust the glucose infusion accordingly. As more tissue is resected and endogenous insulin secretion falls, the blood glucose level will rise if the glucose infusion rate is not decreased.
f. Studies have found no statistically significant difference in intraoperative glucose infusion rates in focal versus diffuse disease (13). Therefore, the form of CHI cannot be determined by the amount of decrease in glucose requirements.
g. Patients can usually be extubated at the end of the surgery unless complications or concomitant disease exists.
h. They usually require intensive care management because of the risk of glucose instability and the need for frequent blood glucose measurement and immediate titration of infusion rates. When total pancreatectomy is needed, these infants will need careful titration of insulin doses, usually initially accomplished with an insulin infusion rather than intermittent subcutaneous dosing (15).
III. Thyroid disease
A. Thyroid hormones play a critical role in normal growth, metabolism, nervous system development, and organ function (16). Disorders of thyroid function can result in permanent neurologic damage, especially in infancy when the thyroid system is immature.
1. The thyroid is the first gland to develop and can be identified at 16 to 17 days of gestation, however, hormone production does not begin until at least 20 to 24 weeks of gestation. As a result, infants born prematurely have low levels of T4 and thyroid-stimulating hormone (TSH) due to their immature hypothalamic–pituitary–thyroid axis.
a. This is usually transient and resolves spontaneously 6 to 10 weeks after birth.
b. Thyroxine-binding globulin (TBG) and T3 levels are also reduced, leading to immature thermogenesis of brown adipose tissue.
c. Treatment with L-thyroxine has been successful in premature infants with severe low birth weight.
2. Unless emergency surgery is required, time should be taken to correct children with thyroid disease to a euthyroid state preoperatively because potentially life-threatening problems can develop under anesthesia with both uncorrected hypo- and hyperthyroidism.
a. Congenital hypothyroidism is characterized by large fontanelles and diastasis of the cranial sutures. An umbilical hernia is often present, and the tongue is large, potentially causing upper airway obstruction. These infants are lethargic and often developmentally delayed. They are disposed to bradycardia, hypotension (with a narrow pulse pressure), and hypothermia. In severe cases, heart failure may be present due to myxomatous changes in the myocardium. Decreased intravascular volume, increased systemic vascular resistance, and blunted baroreceptor reflexes are also seen.
b. There is an increased prevalence of hypothyroidism in individuals with Down syndrome. It can be clinical or subclinical (increased plasma TSH with normal thyroid hormone concentration) (17). Early childhood prevalence rates of any thyroid dysfunction have been estimated to be 15% in individuals with Down syndrome with a lifetime prevalence rate of hypothyroidism ranging from 13% to 63%. Additional syndromes and birth conditions associated with hypothyroidism include Beckwith–Wiedeman syndrome, Bamforth–Lazarus syndrome, Pendred syndrome, and multiple births.
c. Acquired hypothyroidism in childhood can occur after bone marrow transplantation, radiation therapy, following cardiopulmonary bypass, and in critical illness (18,19). Malignancies are rare, but can occur, especially in the MEN type 2 syndrome (20,21).
d. Hypothyroid patients tend to have an exaggerated response to surgical stimulation and may become hemodynamically unstable during anesthesia. Impaired drug metabolism may be seen, which can lead to organ toxicity. They can have an exaggerated response to sedative drugs and may have delayed emergence from anesthesia, although minimum alveolar concentration (MAC) does not appear to be altered.
e. Hyperthyroidism is rare in infancy and is most commonly seen in adolescents with Graves disease (22).
(1) These children exhibit the same signs and symptoms seen in adults such as tremors, irritability, tachycardia, hypertension, cardiomegaly, and exophthalmos.
(2) A goiter is often palpable; if large enough, it may compromise the airway either by direct compression and, if long-standing, by tracheomalacia.
(3) “Thyroid storm,” an acute decompensation, may occur intraoperatively and mimic many of the signs and symptoms of malignant hyperthermia, which include fever, tachycardia, and hypermetabolism. This emergency should be treated with hemodynamic management (β-blockade with esmolol) and thyroid suppression with propylhydrouricil, with the assistance of an endocrinologist.
CLINICAL PEARL Thyroid storm and malignant hyperthermia are both urgent and rapidly progressive disorders. While they share several clinical manifestations, malignant hyperthermia presents with metabolic acidosis, severe hypercarbia, muscle rigidity, and elevated creatinine phosphokinase, whereas thyroid storm does not. Proper differentiation is essential as treatment is different for each of the two disorders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree