Endocrine
22-1 Diabetes Mellitus Types 1 and 2
Matthew Goldman
Clinical Presentation
The classic symptoms of diabetes mellitus (DM) are polyuria, polydipsia, and weight loss despite polyphagia.1 Most cases of type 1 DM manifest acutely and are diagnosed shortly after disease onset. These patients are often metabolically unstable and progress to diabetic ketoacidosis if left untreated.1 Type 2 DM manifests in a much more insidious manner.1 It is important to remember that the first presentation for DM may be ketoacidosis.
Pathophysiology
In type 1 DM, pancreatic beta-islet cells are destroyed (probably via autoimmune mechanisms), resulting in rapid loss of insulin secretion. The origin of type 2 DM involves impaired insulin secretion, increased hepatic glucose production, and decreased muscle glucose uptake.1 Screening for type 2 DM is recommended, because the onset of disease often precedes clinical diagnosis by 10 to 12 years, and 50% of patients have complications by the time the diagnosis is established.1
Diagnosis
Diagnosis of DM is made by the finding of a random glucose concentration greater than or equal to 200 mg/dL, a fasting plasma glucose value greater than or equal to 126 mg/dL, or a 2-hour glucose value on 75-g oral glucose tolerance testing greater than or equal to 200 mg/dL.1 This test should be repeated on a separate day for firm confirmation.1
Clinical Complications
Complications can be macrovascular in nature (e.g., cardiovascular plaque formation), or they may entail microvascular diseases (e.g., diabetic retinopathy, neuropathy, nephropathy).1 Tight glycemic control is effective in controlling microvascular disease progression, but the evidence is not clear regarding macrovascular complications.1 Diabetic retinopathy is a leading cause of blindness, diabetic nephropathy is a common cause of end-stage renal failure, and diabetic neuropathy is a chronic debilitating complication that can be quite painful.1
Management
Type 1 DM is treated with exogenous insulin, using a variety of preparations and injection schedules.1 The treatment of type 2 DM ranges from diet and exercise modification to the use of an assortment of oral medications.1 Combination regimens of oral hypoglycemic agents are recommended for patients with hard-to-control DM.1 Type 2 diabetics may require exogenous insulin if oral medications fail to maintain euglycemia.1
REFERENCES
1. Weiland D, White R. Diabetes mellitus. Clin Fam Pract 2002;4:703.
22-2 Hyperosmolar Nonketotic Coma
Mamie Caton
Clinical Presentation
Hyperosmolar nonketotic coma manifests with severe dehydration, polydipsia, polyuria, altered mental status, tachycardia, hypotension, and weight loss.1 Complaints mimicking neurologic diseases, including stroke, seizure, and, in very severe cases, coma, have been reported1 (see Table 22-2).
Pathophysiology
Hyperosmolar nonketotic coma, also known as hyperglycemic hyperosmolar syndrome (HHS), is a diabetic emergency condition in which hyperglycemia and hyperosmolality result in profound dehydration.1,2,3 Severe hyperglycemia leading to profound osmotic diuresis is usual.1 Glucose-induced diuresis leads to hypovolemia and a stress-response release of the insulin counterregulatory hormones, such as growth hormone, epinephrine, and, above all, glucagon.1 The severe hypovolemia causes a decreased glomerular filtration rate (GFR) and the inability to excrete glucose, worsening the hyperglycemia.2 Because these patients are usually type 2 diabetics, they do make a small amount of insulin, which is enough to prevent lipolysis and diabetic ketoacidosis.2
Diagnosis
Diagnosis is based on the measurement of serum osmolality and the glucose level. Glucose readings are usually greater than 600 mg/dL, and the serum osmolality is greater than 320 mOsm/dL. The pH should be greater than 7.3.1
TABLE 22-2 Clinical Characteristics Associated with Hyperosmolar Nonketotic Coma | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Clinical Complications
The mortality associated with HHS has been divided into early (before 72 hours) and late (after 72 hours) types. Early causes of mortality include shock, sepsis, and death from the underlying cause. Late mortality is most frequently caused by a large-vessel thromboembolic event such as an infarct (cerebral or myocardial), pulmonary embolism, mesenteric vessel thrombosis, or disseminated intravascular coagulopathy.1,2,3
Management
The highest treatment priority is restoring circulatory volume. The mean total body water deficit seen in patients with HHS is approximately 9 L.2 Although normal saline is relatively hypotonic in the HHS patient, some clinicians recommend administration of 50% normal saline until the osmolality is less than 320 Osm/dL.1 Restoration of volume causes glucose levels to drop even in the absence of insulin, because of increased GFR. Therefore, HHS patients tend to respond quickly to insulin while fluid restoration is occurring. Potassium is the most common serious electrolyte imbalance requiring replacement. Because of the high risk of thromboembolism, prophylaxis with low-dose heparin has been recommended.1
REFERENCES
1. Magee MF, Bhatt BA. Management of decompensated diabetes: diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Crit Care Clin 2001;17:75-106.
2. Matz R. Management of hyperosmolar hyperglycemic syndrome. Am Fam Physician 1999;60:1468-1476.
3. Pettigrew DC. Index of suspicion, case 2. Diagnosis: hyperglycemic nonketotic hypertonicity. Pediatr Rev 2001;22:169-173.
22-3 Diabetic Ketoacidosis
Mamie Caton
Clinical Presentation
Early symptoms of diabetic ketoacidosis (DKA) include those of hyperglycemia, such as polyuria, polydipsia, and fatigue.1 Patients may develop more severe signs or symptoms, such as vomiting, tachycardia, tachypnea, Kussmaul’s respirations, and hypotension, as acidosis, ketosis, and dehydration develop. The breath may have the distinct “fruity odor” of acetone. Laboratory evaluation reveals an anion gap acidosis, as well as various electrolyte abnormalities.1,2
Pathophysiology
DKA may be triggered by stressors such as sepsis, pancreatitis, myocardial infarction, or surgery, or it may develop in the setting of new-onset diabetes mellitus. In 2% to 10% of cases, no inciting cause can be elicited2 (see Table 22-3A). Lack of insulin effect, due to either the absence of insulin or the loss of insulin receptor response, leads to decreased peripheral glucose utilization, resulting in serum hyperglycemia and cellular hypoglycemia. Serum hyperglycemia is further exacerbated by increases in hepatic glucogenesis and glycogenolysis. Fatty acid oxidation in the adipocytes leads to ketone production and acidosis. Hyperglycemia may lead to an osmotic diuresis and dehydration as the active reabsorption of glucose in the kidney becomes saturated.1,2
TABLE 22-3A The “Four I’s” That Cause Diabetic Ketoacidosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
TABLE 22-3B Treatment Modalities for Diabetic Ketoacidosis | ||||||
|---|---|---|---|---|---|---|
|
Diagnosis
Diagnosis is based on serum acidosis and ketosis in the presence of elevated glucose and low bicarbonate levels. The glucose concentration is usually greater than 250 to 300 mg/dL, and the pH should be less than 7.35.2
Clinical Complications
Complications of DKA include thromboembolism, gastroparesis, rhabdomyolysis, seizures, coma, and death.2 Complications of treatment may include fluid overresuscitation (cerebral edema, pulmonary edema, and acute lung injury), overcorrection of hyperglycemia (hypoglycemia), and electrolyte abnormalities (hypokalemia and hypophosphatemia).1,2
Management
The goals of treatment are to eliminate the cause, the ketosis, and the acidosis, and to restore circulatory volume along with electrolyte imbalances (see Table 22-3B). Glucose levels should be checked hourly and the rate of insulin adjusted accordingly. Electrolytes should be monitored at least every 2 hours during insulin infusion. If ketosis persists despite normoglycemia, the insulin drip should be continued with supplemental glucose administration to avoid hypoglycemia.1,2
REFERENCES
1. Rosenbauer J, Icks A, Giani G. Clinical characteristics and predictors of severe ketoacidosis at onset of type 1 DM in children in the North Rhine Westphalian region. J Pediatr Endocrinol Metab 2002; 15:1137-1145.
2. Magee MF, Bhatt BA. Management of decompensated diabetes. Crit Care Clin 2001;17:75-106.
22-4 Myxedema Coma
Jay Itzkowitz
Clinical Presentation
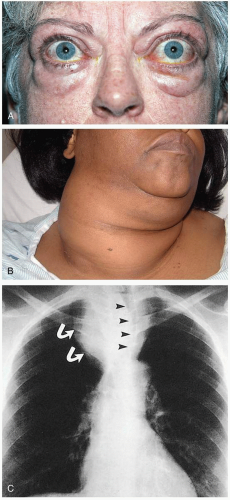
A history of hypothyroidism should be sought in any comatose patient with a history of hypothermia and respiratory failure. However, evidence of hypothyroidism may be minimal or absent. Most patients present with hypothermia.1 Respiratory failure is common and is characterized by hypoxia, hypoventilation, and hypercarbia.2 Hypotension and bradycardia are also common presentations. The patient can have a distended abdomen secondary to an ileus or ascites. As the name suggests, patients have cold, nonpitting edema of the hands and feet. Patients also present with some alteration of their mental status.
Pathophysiology
The single most important factor in the evolution of hypothyroidism to myxedema coma is physiologic stress.3 Congestive heart failure and pulmonary infections are the most common causes. Drugs (phenothiazines, narcotics, β-adrenergic blockers, lithium, and iodide), trauma, exposure to cold, infection, and hemorrhage may also be precipitants. Myxedema coma is most prevalent in elderly women and usually occurs during the winter months.3
Diagnosis
Although thyroid function tests are the only way to diagnose a thyroid problem with certainty and should be ordered, the results are not always available in a timely fashion. The diagnosis of myxedema coma should be entertained if an elderly patient presents with altered sensorium, hypothermia, and an array of physical and metabolic abnormalities.1,2,3
Clinical Complications
Without treatment, the major life-threatening complications of myxedema are hypotension, respiratory insufficiency, and coma.
Management
Supportive measures are essential. For the patient who presents with hypothermia, slow rewarming should be undertaken. The use of warming blankets should be avoided, because the peripheral dilatation may worsen the hypotension.3 Electrolyte abnormalities (e.g., hyponatremia) should also be corrected. Patients who are hypoglycemic should be treated with 50% dextrose (D50) in water. If an infection source is found, it should be treated with appropriate antibiotics. Treatment with thyroid hormone is the most critical aspect of therapy. Because gastrointestinal absorption is compromised in myxedema, intravenous therapy is indicated. The drug of choice is intravenous thyroxine.3
REFERENCES
1. Olsen CG. Myxedema coma in the elderly. J Am Board Fam Pract 1995;8:376-383.
2. Nicoloff JT, LoPresti JS. Myxedema coma: a form of decompensated hypothyroidism. Endocrinol Metab Clin North Am 1993;22:279-290.
3. Wall CR. Myxedema coma: diagnosis and treatment. Am Fam Physician 2000;62:2485-2490.
22-5 Hypothyroidism
Matthew Goldman
Clinical Presentation
Hypothyroidism usually has an insidious onset, with patients commonly complaining of fatigue, cold intolerance, generalized weakness, constipation, and depression.1
Pathophysiology
The most common cause of hypothyroidism worldwide is iodine deficiency, but in the United States it is Hashimoto’s (chronic autoimmune) thyroiditis.1 Primary hypothyroidism results from failure of the thyroid gland to produce the thyroid hormones triiodothyronine (T3) and thyroxine (T4).1 Secondary hypothyroidism results from a decrease in circulating thyroid-stimulating hormone (TSH), a hormone produced in the anterior pituitary gland that stimulates the thyroid gland to become active.1 Tertiary hypothyroidism results from decreased thyrotropin-releasing hormone (TRH) levels secondary to hypothalamic insufficiency.1
Diagnosis
Hypothyroidism may be suspected clinically, although laboratory studies are the primary modalities used to make the diagnosis.1 Once decreased thyroid hormone levels are confirmed, the TSH level is the most important factor to determine whether the hypothyroidism is primary or secondary. Radiographic imaging studies and tissue sampling may provide additional confirmatory information, although they generally are not needed once laboratory confirmation has been attained.1
Clinical Complications
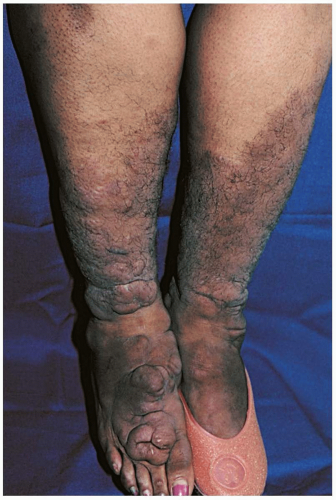
Myxedema coma is the most common complication of hypothyroidism; it usually occurs during the winter months, when thermoregulatory stressors are at maximum levels.1 Common precipitants include hypothermia, trauma, burns, surgery, strokes, sepsis, and medications. Cardinal findings are hypothermia and altered mental status, in addition to bradycardia, hypotension, hypoventilation, and hyponatremia.1 If present, myxedema is characterized by generalized skin and soft-tissue swelling, often with associated periorbital edema, ptosis, and macroglossia.1
Management
Once laboratory testing has confirmed hypothyroidism, levothyroxine should be administered.1 If the hypothyroidism is secondary to pituitary failure, glucocorticoids should also be given.1 Serum T4 levels usually return to normal within 1 to 2 weeks, and TSH levels normalize within an additional 4 to 6 weeks.1
REFERENCES
1. Wilson G. Thyroid disorders. Clin Fam Pract 2002;4:667.
22-6 Hyperthyroidism
Jay Itzkowitz
Clinical Presentation
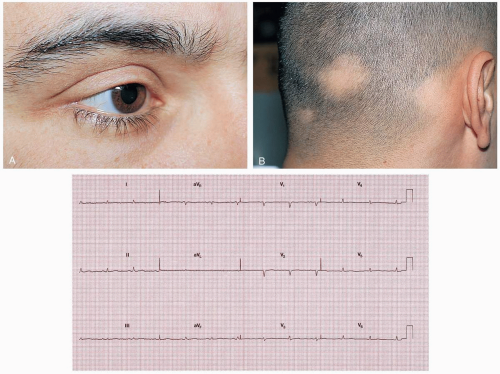
Symptoms of hyperthyroidism include palpitations, nervousness, weight loss despite increased appetite, excessive sweating, hyperdefecation, and heat intolerance.1 Women may also report a decrease in or cessation of menses.2 Common presenting findings include atrial fibrillation, lid lag with a stare, fine tremor, muscle weakness, and unexplained tachycardia.1
Pathophysiology
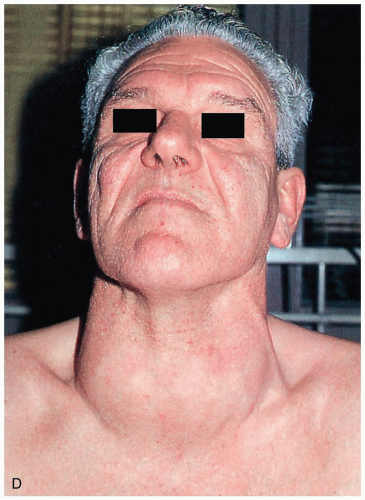
The hypermetabolic state that is caused by excess circulation of the thyroid hormones triiodothyronine (T3) and thyroxine (T4).1 Hyperthyroidism involves a disruption of the homeostatic mechanisms that normally control hormone secretion.1 These include primary (thyroid), secondary (pituitary), and tertiary (hypothalamus) disorders. The most common cause of hyperthyroidism is Graves’ disease (toxic diffuse goiter), followed by toxic multinodular goiter.1
Diagnosis
Patients presenting with palpable goiter, exophthalmos, and pretibial myxedema are assumed to have Graves’ disease. Mild cases of hyperthyroidism are difficult to diagnose because the symptoms are vague. The most reliable screening measure of thyroid function is the thyroid stimulating hormone (TSH) concentration.3 In patients with primary hyperthyroidism, the TSH levels are suppressed and the T3 and T4 levels are elevated. Patients with secondary hyperthyroidism have elevated TSH, T3, and T4 levels.3
Clinical Complications
Cardiac manifestations including high-output congestive heart failure and atrial arrhythmias may occur. Exposure keratitis can result from constant staring without blinking and lid lag. If undiagnosed or left untreated, hyperthyroidism may evolve into thyroid storm, a life-threatening condition.
Management
Mild hyperthyroidism does not require immediate treatment in the emergency department. Patients may be referred to an outpatient endocrinologist for further evaluation. Mild to moderate symptoms can be alleviated with β-adrenergic blockers. Antithyroid medication such as propylthiouracil or methimazole may also be started. Definitive therapy includes a partial or total thyroidectomy, accomplished either surgically or medically with radioactive iodine.
REFERENCES
1. Wilson G. Thyroid disorders. Clin Fam Pract 2002;4:667.
2. Bryer-Ash M. Evaluation of the patient with a suspected thyroid disorder. Obstet Gynecol Clin North Am 2001;28:421-438.
3. de los Santos ET, Starich GH, Mazzaferri EL. Sensitivity, specificity, and cost-effectiveness of the sensitive thyrotropin in the diagnosis of thyroid disease in ambulatory patients. Arch Intern Med 1989;149:526-532.
22-7 Thyroid Storm
Jay Itzkowitz
Clinical Presentation
Thyroid storm (TS) manifests as severe hyperthyroidism with concomitant central nervous system hyperactivity, including anxiety, restlessness, manic behavior, and emotional lability (see Table 22-7). Patients with TS may mistakenly be thought to have a psychiatric disorder.1,2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree