KEY POINTS
Encephalomyelitis should be suspected in patients with neurologic findings including fever, headaches, odd behavior, altered sensorium, seizures, and focal neurologic deficits without obvious cause.
Course of symptoms, season of the year, travel, local outbreaks, occupation, animal or insect exposure, recent illness, immune status, age, and recent vaccine history all play a part in trying to establish a diagnosis. Physical examination is not usually revealing, but rashes, inoculation reactions, or pneumonia can be helpful clues.
An individualized workup based on the above includes culture, PCR, antigen detection, serologic IgM and IgG titers of noncentral nervous tissue, as well as cerebrospinal fluid (CSF) analysis if not contraindicated by examination. The results must be used carefully based on the context of the presentation.
CSF is almost always abnormal but a normal study does not preclude disease. A positive PCR or antigen study should help guide treatment. Cell counts in the CSF can be clues to nonviral causes (eg, eosinophilia with parasites and coccidioidomycosis).
Magnetic resonance imaging (MRI) is a key part of the workup and is more reliable than CT scan. Either of these tests should be obtained prior to lumbar puncture if possible.
Herpes simplex virus (HSV) is the most common cause of nonepidemic fatal encephalitis. Early treatment with acyclovir significantly reduces mortality.
Arboviruses are the most common cause of epidemic outbreaks worldwide and depending on the area and season may be a clue to the origin.
In all suspected cases of encephalomyelitis treatment with full-dose acyclovir should begin quickly until a diagnosis is established. A negative workup does not preclude HSV so empiric therapy is appropriate.
Supportive care, along with appropriate therapy, is essential because some patients recover even following protracted illness.
Consider bioterrorism in unexplained outbreaks, especially when the presentation is unusual or out of season.
INTRODUCTION
Encephalomyelitis is a combination of two disease states affecting the central nervous system (CNS). Encephalitis is defined as an inflammatory process of the brain associated with neurologic dysfunction; myelitis is an inflammatory process affecting the spinal cord. These may occur separately or together. Occasionally the covering of the brain is involved and therefore the term meningoencephalitis or meningoencephalomyelitis may be more appropriate.
There are about 20,000 cases of encephalomyelitis reported per year. The approach to each suspected case of encephalomyelitis should be standard yet individualized based on that patient’s clinical presentation. The course of symptoms, season of the year, travel, local outbreaks, occupation, animal or insect exposure, recent illness, immune status, age, and recent vaccine history all play a part in establishing a diagnosis. Physical examination is not usually revealing, but rashes, inoculation reactions, or pneumonia can be helpful clues.
Though viruses are the predominant cause of encephalomyelitis, autoimmune and acute disseminated encephalomyelitis (ADEM) may account for approximately 20% of cases. Other noninfectious causes of this syndrome must also be excluded, such as vasculitis, connective tissue diseases, and paraneoplastic syndromes.
A clinical correlation between non-CNS infections and CNS manifestations is not always clear or evident. An individualized workup includes culture, PCR, antigen detection, and serologic IgM and IgG titers of noncentral nervous tissue (ie, sputum, stool, blood, and nasopharyngeal swabs). CSF analysis is of great value if lumbar puncture is not contraindicated by findings on clinical examination or imaging. The results must be used carefully based on the context of the presentation.
CSF is almost always abnormal but a normal study does not preclude disease. A positive PCR or antigen study should help guide the treatment. Cell counts in the CSF can be clues to nonviral causes (eg, eosinophilia with parasites and coccidioidomycosis).
Magnetic resonance imaging (MRI) is a key part of the workup, is more reliable than CT, and should be obtained prior to lumbar puncture if possible. If MRI cannot be done or is impractical, CT with and without contrast can be helpful. PET, EEG, and brain biopsy are not usually required. MRI or CT can occasionally be diagnostic or help guide the workup in other directions. In the presence of a raised intracranial pressure, a cisternal puncture can be done to obtain CSF. EEG can be useful in patients with persistent altered mental status to evaluate for nonconvulsing status epilepticus and temporal activity associated with herpes simplex encephalitis.
Despite all efforts to obtain a diagnosis, most cases of meningoencephalitis remain cryptic. Arboviruses are the most common epidemic causes of encephalomyelitis; they are usually preceded by zoonotic outbreaks, that is, chickens in St Louis encephalitis, birds in West Nile, and horses in Eastern Equine Encephalitis.
Herpes simplex virus (HSV) is the most common cause of nonepidemic fatal encephalitis. If untreated, mortality is approximately 60% to 80%; early treatment with acyclovir significantly reduces mortality to approximately 10% to 20%. All suspected cases of encephalomyelitis should be treated with acyclovir while the results of the workup are pending. In addition, antibiotics, antifungals, and antituberculosis medications may be appropriate based on the clinical presentation. In patients with ADEM, corticosteroids should be considered, as well as plasma exchange if the patient’s condition continues to deteriorate. The care of these patients is mostly supportive and recovery can occur after long and even extreme changes in mental function.
A wide range of viruses, bacteria, fungi, and parasites are associated with encephalitis, though viruses are the most common etiology. See Table 72-1 for the most common causes of encephalitis, and Table 72-2 for the less common causes, as well as diagnostic and treatment information.
Common Causes of Encephalitis
|
Less Common Causes of Encephalitis, Diagnosis and Treatment Summary
| Etiology | Epidemiology | Clinical Features | Diagnosis | Treatment |
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HERPES VIRIDAE
Viruses that are associated with encephalomyelitis in the Herpesviridae family are herpes simplex virus 1 and 2 (HSV-1 and HSV-2), varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus 6 (HHV-6), and herpes B virus. Viral shedding occurs in symptomatic and asymptomatic persons infected with HSV, CMV, EBV, and HHV-6 and can be transmitted by virus-containing body fluids to susceptible hosts.
Herpes simplex virus type 1 (HSV-1) encephalitis is the most common recognizable cause of sporadic fatal encephalitis worldwide. Early recognition is of utmost importance due to its significant mortality and morbidity.1,2 Treatment with acyclovir should be initiated early even with minimal clinical suspicion.
Epidemiology and Pathogenesis: Herpes simplex virus encephalitis (HSVE) is the most common cause of fatal nonepidemic encephalitis. It is estimated to affect at least 1 in 500,000 individuals per year.1 HSVE occurs in all age groups, with a bimodal peak distribution in patients younger than 20 years and older than 50 years of age. It occurs equally in both sexes and has no racial predilection. This is seldom a disease of the immunocompromised. Indeed, it is hard to predict who is at risk for HSVE.3
HSV infection of the central nervous system arises from either primary infection or reactivation. Primary infection, which is most common in children, occurs due to direct CNS invasion via the olfactory tract following HSV infection of the oropharynx. Alternatively, latent infection most common in adults >50 years occurs due to viral reactivation in the trigeminal ganglia and reaches the CNS along a neurotropic route. HSVE is the most common clinical presentation of HSV and has a characteristic temporal lobe predilection. In most cases, necrosis of the temporal lobes is seen with associated clinical deficits. Much of the pathogenesis of HSVE seems to be immune mediated.4,5
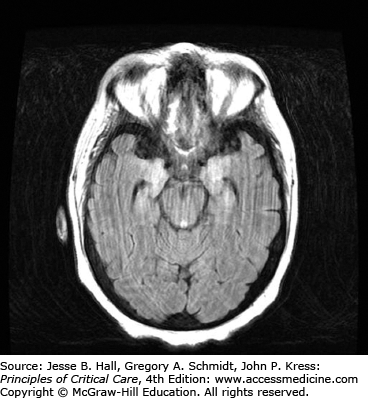
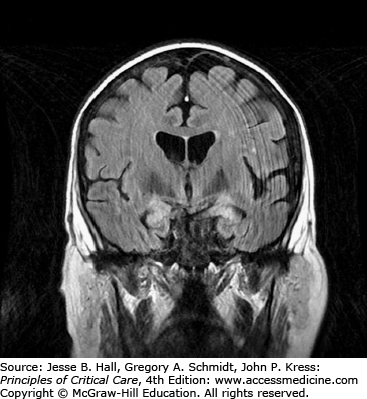
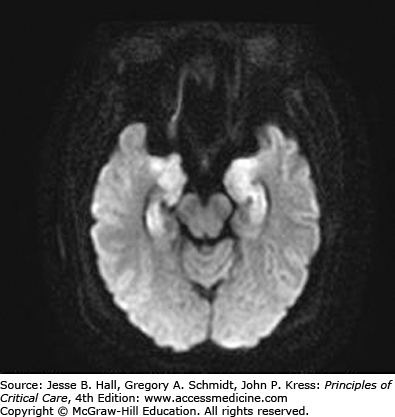
Clinical Features and Diagnosis: There are no typical clinical features of HSVE. The clinical syndrome is often characterized by acute onset of fever, headache, seizures, focal neurologic deficits, and altered mental status. There may be an influenza-like prodrome. CSF examination typically shows lymphocytic predominance, elevated protein, and normal glucose levels. Red blood cells are present in 75% to 80% of samples, indicating the hemorrhagic nature of this encephalitis.6 Unilateral temporal lobe abnormalities on brain imaging are characteristic for HSVE. CT scans have only 50% sensitivity early in the course of illness,3 but can be performed to exclude raised intracranial pressure prior to lumbar puncture. Abnormalities include localized edema, low-density lesions, mass effect, contrast enhancement, and hemorrhage. On the other hand, MRI is the most sensitive and specific neurodiagnostic test for HSVE and has excellent delineation of the temporobasal lobe of the brain (Figs. 72-1 and 72-2). Diffusion-weighted MRI is especially preferred early in the course of the disease (Fig. 72-3).7,8 MRI shows abnormal signal earliest in FLAIR sequences and diffusion-restricted images of the medial temporal lobes and insulas. Brain perfusion SPECT scan shows increased tracer accumulation in the affected temporal lobe with high specificity.9 Focal EEG findings occur in 80% to 90% of cases of HSVE, but are nonspecific. The EEG typically shows focal spiked and slow wave (theta and delta slowing) patterns localized to the area of the brain involved. Paroxysmal lateralized epileptiform discharges are also seen but are nonspecific. PCR analysis to detect HSV DNA in CSF is the gold standard for establishing the diagnosis of HSVE, especially in the early phase of the disease. It has a sensitivity of 98% and a specificity of 99%. In very early cases, PCR can be negative and it also becomes negative by the second or third week of the disease.10,11 Treatment of HSVE should be initiated while awaiting PCR results. Later in the course of the disease, HSV antigen and antibody become positive. Brain biopsy, should only be considered in very select circumstances, when the diagnosis remains uncertain despite all available investigations and particularly when the patient is deteriorating clinically despite empiric therapy.12 Biopsy specimens are tested for presence of HSV by culture, for HSV antigens by immunohistochemistry, and for viral DNA by in situ hybridization. Pathology shows perivascular cuffs of lymphocytes and numerous small hemorrhages.
Management: HSVE is a devastating infection, with significant mortality and a grim prognosis. Even with treatment two-thirds of the survivors end up with significant neurologic deficits.2 Empiric therapy should be initiated even with minimal clinical suspicion before the onset of dominant temporal lobe hemorrhagic necrosis and significant deterioration of consciousness. The recommended treatment of HSVE is acyclovir at a dose of 10 mg/kg every 8 hours for 14 to 21 days. It prevents viral replication by inhibiting the viral (as well as the cellular) DNA polymerase in infected cells. Introduced in the mid-1980s, it replaced vidarabine and was shown to reduce mortality from 70% to 20%.13,14 Relapses occur commonly in children within 2 weeks of completing antiviral therapy necessitating a second course of acyclovir.15 Acyclovir has renal and neurologic toxicity manifesting as crystalluria, renal failure, and encephalopathy. Effective hydration, dose adjustment, or discontinuation of therapy is helpful. Patients receiving acyclovir for suspected HSVE should also receive other broad-spectrum antibiotics for the first 48 to 72 hours until CSF and other cultures for bacteria are negative. The time period that HSV DNA can be detected in the CSF once therapy has been initiated is unclear. Discontinuation of therapy based on negative CSF PCR results depends on the clinical probability of HSVE and is a matter of judgment.16 Use of corticosteroids is controversial though a few studies have shown favorable outcomes.17
In spite of effective treatment, mortality is still up to 20% to 30%.3 The precise factors that determine the therapeutic response are unknown. Patients with low initial level of consciousness and age over 30 have a very poor prognosis in general. Long-term complications of HSVE infection are common and include neurocognitive impairment, residual dysphasias, paresis, paresthesias, behavioral changes, and a Korsakoff-like amnesia.18
HSV-2 encephalitis is seen primarily in neonates, where brain involvement is generalized and is usually acquired during vaginal delivery from asymptomatic infected mothers. Neonatal encephalitis is associated with serious mortality and morbidity.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree