Fig. 7.1
A management algorithm for parapneumonic effusion. Reproduced with permission from Moore et al. [63, Fig. 1], with permission courtesy of Wolters Kluwer Health, Inc
After tube placement, vigilant follow-up of chest tube output and changes in radiographic appearance are critical. Pleural collections persisting for more than 24 h warrant prompt computed tomographic (CT) imaging for evaluation of the entire thoracic space [12, 32]. Delay in diagnosis of an undrained simple fluid collection allows progression to a complex multi-locular process and the final organization stage [37]. As Sahn and Light [31] stated in 1989, “the sun should never set on a parapneumonic effusion”; early diagnosis and treatment of complicated pleural infection is essential for optimal outcomes. CT images are crucial for the next step in the management of pleural space infections that have not resolved with tube drainage as this dictates operative versus fibrinolytic therapy (Fig. 7.2).
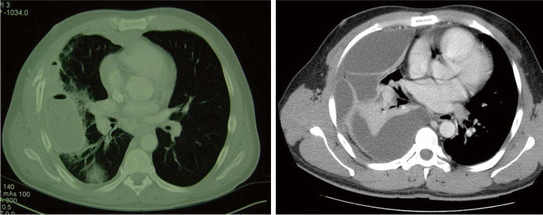

Fig. 7.2
CT imaging distinguishes a simple pleural collection (left) that may respond to fibrinolytic therapy versus a complex pleural collection that warrants prompt VATS. Reproduced with permission from Moore et al. [63, Fig. 2], with permission courtesy of Wolters Kluwer Health, Inc
Fibrinolysis Therapy for Treating Pleural Space Infections
The rationale for obtaining a CT scan 24 h after failure for appropriate tube drainage of a pleural infection is for recognition of a persistent pleural collection trapped via thin fibrin septa. This fibrin deposition likely has an initial protective role. The prehistoric horseshoe crab uses a unique protease, Factor C, to initiate coagulation in the presence of endotoxin, trapping and killing pathogens [38]. This has been extrapolated to animal models, in which it has been demonstrated that antifibrinolysis is protective in Gram-negative infection [39]. However, excessive fibrin deposition maybe pathologic and impaired fibrinolysis recently been described ventilated patients [40]. It has become increasingly apparent that the majority of patients in sepsis [41] or sustaining significant injury [42] have resistance to fibrinolytic activity and prone to developing organ failure from what is believed to be microvascular fibrin deposition. This translates to the pleural space, where fibrin deposition is appreciated during inflammation and infection [43]. This early fibrin deposition may help contain the pathogen with impending progressive infection. Pathologic fibrin deposition occurs when the body is unable to clear the pathogen, and the septa become thickened, rendering chest tube drainage ineffective. The proposed therapeutic option enzymatically breaks down these fibrin depositions by up-regulating the fibrinolytic system.
The first report of fibrinolytic therapy in the pleural space was given by Tillett and Sherry [44] in 1949. They infused purified hemolytic streptococcal concentrates, presumed to contain streptokinase and deoxyribonuclease (DNase). Although apparently safe, there was no documented improvement in patient outcome during the ensuing 60 years. The first randomized trial, by Davies et al. [45] in 1997, demonstrated radiographic improvement in 24 patients but no discernible clinical benefit. This was followed by a number of underpowered randomized studies in Europe, suggesting that urokinase demonstrated a therapeutic value [46, 47]. These conflicting results led to the MIST I study [48] involving 52 hospitals in the UK with 412 randomized patients. The data indicated that 72 h of streptokinase treatment resulted in no improvement in mortality, rate of surgery, or length of stay and was associated with an increased rate of serious adverse events. This study was criticized for including a heterogeneous mix of patients with different comorbidities and different stages of pleural disease [49]. A subsequent Cochrane review in 2008 [50] noted that there was a discordance between earlier studies and the MIST I data and concluded that fibrinolytics should be used selectively because there has not been a proven benefit in high-quality trials; however, the authors acknowledged that there may be certain subgroups of patients who benefit from this therapy. Clinical studies in other arenas indicated that tissue plasminogen activator (tPA) was a more effective and safer agent than streptokinase or urokinase as a fibrinolytic agent [51]. Other studies suggested that the addition of DNase to streptokinase improves evacuation of an empyema [52, 53]. Subsequently, MIST II, using tPA with or without DNase, has been completed [54]. Unfortunately, this study (n = 210; four study groups) was only powered sufficiently to evaluate radiographic changes. But consistent with MIST I, tPA alone had no benefit over no fibrinolytic treatment. The combination of tPA and DNase, however, was beneficial in both the primary end point (radiographic clearance) and secondary end points (need for thoracotomy, hospital length of stay). The authors responsibly conclude, “Our study shows that combination intrapleural t-PA and DNAse therapy improves the drainage of pleural fluid in patients with pleural infection… This combined treatment may therefore be useful in patients in whom standard medical management has failed and thoracic surgery is not a treatment option. However, appropriate trials are needed to accurately define the treatment effects.”
Thus, the debate continues regarding the role of fibrinolytics in the management of pleural collections. Most intensivists have observed effective eradication of early empyema in some patients, but agree that the appropriate population remains to be defined. On the basis of the pathophysiology of empyema and the morbidity of thoracotomy for delayed intervention, most intensivists believe that fibrinolytic treatment should be attempted for an early empyema with simple collections separated by thin septa documented by CT scan if tube thoracotomy drainage fails (Fig. 7.2). Image-guided direct infusion of fibrinolytics into the collection is superior to delivery via the failed chest tube. The precise agent, dosage, and timing of infusion remain to be determined, although the combination of tPA and DNase seems to be the most effective regimen at this time [54]. Large case series have emerged since the publication of MIST II. Piccolo et al. [55] reported a three-year experience using the MIST II protocol (5 mg DNase and 10 mg tPA BID for up to six doses) in 10 different centers. Inclusion criteria were patients with a pleural pH < 7.2 and clinical evidence of infection. The majority of these patients were male (69 %) had CAP (97 %), middle aged (median 56 years), and received 2 days of therapy. Of the 107 patients included in the analysis eight (93 %) had successful fibrinolytic/dnase therapy. Of note 23 % of patients had increased pain associated with infusion and required additional analgesic medication, which should be taken into consideration when starting therapy. A smaller case series from Mehta et al. [56] evaluating 55 patients using once-a-day therapy for 3 days had similarly positive results with 93 % of patients not requiring surgical intervention. They also appreciated that a decent number of patients (15 %) required additional analgesics during treatments.
Surgical Decortication
There are no randomized control trials evaluating VATs versus fibrinolytic therapy in adults. However, in adolescents a small trial demonstrated equal efficacy in fibrinolytic therapy and VATS in clearing infection, but the surgical intervention group has 3 fewer days with a chest tube and 3 fewer hospital days [57]. This is important to take into the context of the patient’s physiologic status. There is a need to for a prospective randomized control trial to determine whether VATS or fibrinolytic therapy is the optimal treatment of patients with complicated parapneumonic effusions who are physically fit to undergo surgery. In addition, there are also patents who should proceed to decortication and avoid futile attempts at fibrinolysis therapy. Multi-loculated empyemas with an established pleural peel evident on CT scanning should undergo prompt VATS [50]. Although “medical” VATS using local anesthesia has been reported [58], the standard procedure is lateral decubitus positioning with dual lung ventilation to facilitate comprehensive evaluation of the involved pleural cavity and systematic decortication (Fig. 7.3). A key maneuver is to enter the pleural space without injuring the underlying lung because of extensive pleural adhesions. An initial incision in the upper thorax, where the empyema is least developed, is usually the safest strategy. In most of the cases, we have used the existing chest tube site to free the lung for placement of the initial port. With the thoracoscope in position and the lung at least partially deflated, additional working ports are added under direct vision. The sites for these ports are chosen to match the chest wall entrance of the chest tubes after VATS.
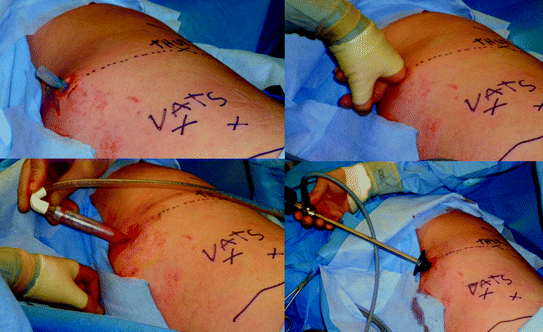

Fig. 7.3
In performing VATS, the thoracic cavity must be entered carefully to avoid tearing the lung because of firm adhesions. We prefer to use the existing chest tube site, digitally mobilizing the adherent lung and further opening a space for the thoracoscope with a large blunt suction tip. Reproduced with permission from Moore et al. [63, Fig. 3], courtesy of Wolters Kluwer Health, Inc
The objectives of VATS are to unroof all loculated collections, including those in the fissures, and to free the lung of the visceral pleural fibrous encasement. Usually, the decortication is initiated in the upper lobe, where the process is more limited, and ultimately, the fibrous debris is removed as much as possible from the lung surface to enable reexpansion. Dissection must be done carefully on the mediastinal side to avoid injury to the phrenic nerve and pulmonary vasculature. Similarly, clearing the diaphragm must be done cautiously to avoid perforation. In fact, the diaphragm does not need to be systematically debrided as long as the lower lobe is freed. After extensive decortication, the thorax is usually drained with three relatively large chest tubes (28F) to facilitate removal of debris and blood associated with the procedure (Fig. 7.4). The most inferior tube is usually an angled tube positioned in the posterior-dependent recess of the chest.
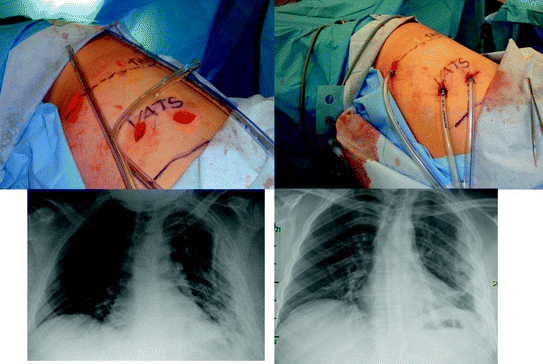

Fig. 7.4
With advanced empyemas, we usually place three relatively large chest tubes; 28F anterior, posterior, and angled above the diaphragm. The chest roentgenogram (left) illustrates the three tube thoracostomies and the follow-up examination at day 6 after sequential removal of the tubes. Reproduced with permission from Moore et al. [63, Fig. 5], with permission courtesy of Wolters Kluwer Health, Inc
In the event of a dense fibrous peel that precludes clearance via VATS, a limited lateral muscle-sparing thoracotomy (“mini thoracotomy”) is performed to accomplish decortication. Transecting the posterior rib facilitates exposure of the fibrous cavity. Advanced empyemas often require scalpel incision to free the lung for reexpansion; inspection of the lung with periodic reinflation should be done to avoid extensive pulmonary parenchymal air leaks. In the unusual case of a chronic empyema, a standard posterolateral thoracotomy is required. Often, the safest approach is to develop an extrapleural plane and directly enter the empyema cavity before any further thoracic dissection is done. After these extensive decortications, the thorax is drained with three relatively large chest tubes (28F), and the most inferior tube is usually an angled tube positioned in the posterior-dependent recess of the chest. Occasionally, these tubes are simply transected to provide external drainage for outpatient management of extended processes.
Treatment of an advanced process caused by a necrotic infected lung with associated major air leaks in a severely immunocompromised patient warrants open thoracic drainage. The Eloesser flap, thoracic cavity marsupialization via segmental rib resection and suturing the skin to the underlying parietal surface, has been the standard for these complicated cases [59]. But recently, simple open drainage with suturing the skin margin to the chest wall, thoracostoma, and the application of a vacuum-assisted wound closure has been popularized [60, 61]. Ultimately, some of these wounds will heal by secondary intention, and the remaining can be closed with thoracomyoplasty [62].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








