Emergency Evaluation and Treatment
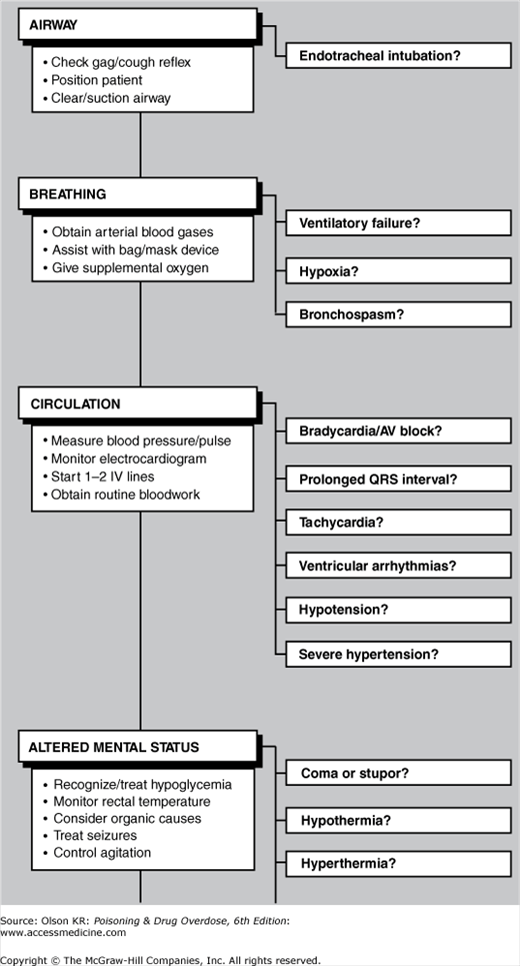
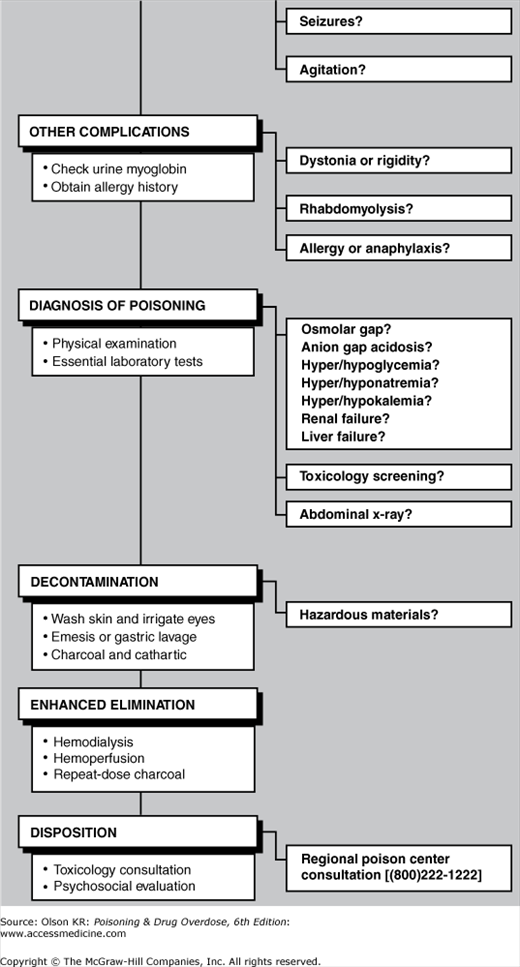
Even though they may not appear to be acutely ill, all poisoned patients should be treated as if they have a potentially life-threatening intoxication. Figure I–1 provides a checklist of emergency evaluation and treatment procedures. More detailed information on the diagnosis and treatment for each emergency step is referenced by page and presented immediately after the checklist.
When treating suspected poisoning cases, quickly review the checklist to determine the scope of appropriate interventions and begin needed life-saving treatment. If further information is required for any step, turn to the cited pages for a detailed discussion of each topic. Although the checklist is presented in a sequential format, many steps may be performed simultaneously (eg, airway management, naloxone and dextrose administration, and gastric lavage).
Assessment. The most common factor contributing to death from drug overdose or poisoning is loss of airway-protective reflexes with subsequent airway obstruction caused by the flaccid tongue, pulmonary aspiration of gastric contents, or respiratory arrest. All poisoned patients should be suspected of having a potentially compromised airway.
Patients who are awake and talking are likely to have intact airway reflexes but should be monitored closely because worsening intoxication can result in rapid loss of airway control.
In a lethargic or obtunded patient, the response to stimulation of the nasopharynx (eg, does the patient react to placement of a nasal airway?) or the presence of a spontaneous cough reflex may provide an indirect indication of the patient’s ability to protect the airway. If there is any doubt, it is best to perform endotracheal intubation (see below).
Treatment. Optimize the airway position and perform endotracheal intubation if necessary. Early use of naloxone (See Naloxone and Nalmefene) or flumazenil (See Flumazenil) may awaken a patient intoxicated with opioids or benzodiazepines, respectively, and obviate the need for endotracheal intubation. (Note: Flumazenil is not recommended except in very select circumstances, as its use may precipitate seizures.)
Position the patient and clear the airway.
Optimize the airway position to force the flaccid tongue forward and maximize the airway opening. The following techniques are useful. Caution: Do not perform neck manipulation if you suspect a neck injury.
Place the neck and head in the “sniffing” position, with the neck flexed forward and the head extended.
Apply the “jaw thrust” maneuver to create forward movement of the tongue without flexing or extending the neck. Pull the jaw forward by placing the fingers of each hand on the angle of the mandible just below the ears. (This motion also causes a painful stimulus to the angle of the jaw, the response to which reflects the patient’s depth of coma.)
Place the patient in a head-down, left-sided position that allows the tongue to fall forward and secretions or vomitus to drain out of the mouth.
If the airway is still not patent, examine the oropharynx and remove any obstruction or secretions by suction, by a sweep with the finger, or with Magill forceps.
The airway can also be maintained with artificial oropharyngeal or nasopharyngeal airway devices. These devices are placed in the mouth or nose to lift the tongue and push it forward. They are only temporary measures. A patient who can tolerate an artificial airway without complaint probably needs an endotracheal tube.
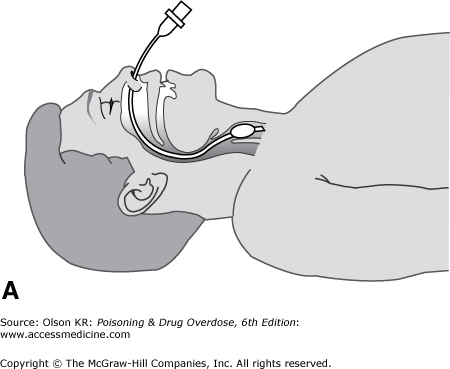
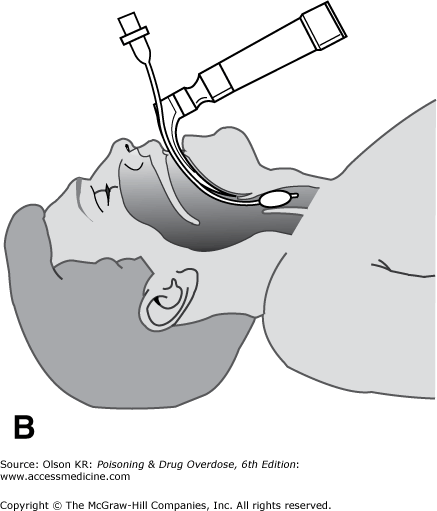
Perform endotracheal intubation if personnel trained in the procedure are available. Intubation of the trachea provides the most reliable protection of the airway, preventing obstruction and reducing the risk for pulmonary aspiration of gastric contents, as well as allowing mechanically assisted ventilation. However, it is not a simple procedure and should be attempted only by those with training and experience. Complications include vomiting with pulmonary aspiration; local trauma to the oropharynx, nasopharynx, and larynx; inadvertent intubation of the esophagus or a mainstem bronchus; and failure to intubate the patient after respiratory arrest has been induced by a neuromuscular blocker. There are two routes for endotracheal intubation: nasotracheal and orotracheal.
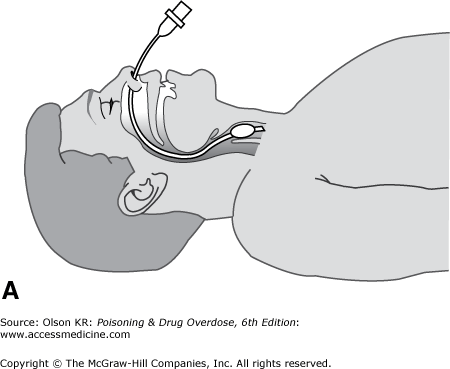
Nasotracheal intubation. In nasotracheal intubation, a soft, flexible tube is passed through the nose and into the trachea by using a “blind” technique (Figure I–2A).
Advantages
It may be performed in a conscious or semiconscious patient without the need for neuromuscular paralysis.
Once placed, it is usually better tolerated than an orotracheal tube.
Disadvantages
Perforation of the nasal mucosa with epistaxis.
Stimulation of vomiting in an obtunded patient.
Patient must be breathing spontaneously.
Anatomically more difficult in infants because of their anterior epiglottis.
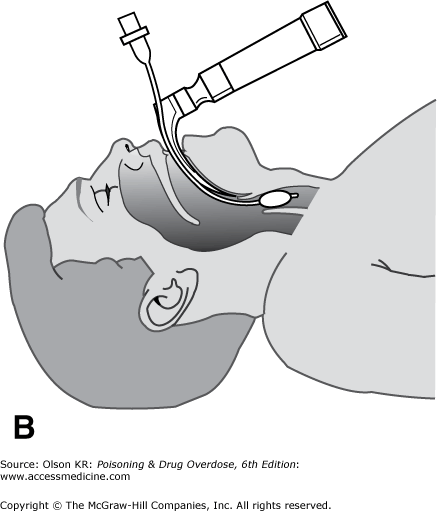
Orotracheal intubation. In orotracheal intubation, the tube is passed through the patient’s mouth into the trachea under direct vision (Figure I–2B) or with the aid of a bougie.
Technique
Advantages
Performed under direct vision, making accidental esophageal intubation less likely.
Insignificant risk for bleeding.
Patient need not be breathing spontaneously.
Higher success rate than that achieved via the nasotracheal route.
Disadvantages
Frequently requires neuromuscular paralysis, creating a risk for fatal respiratory arrest if intubation is unsuccessful.
Requires neck manipulation, which may cause spinal cord injury if the patient has also had neck trauma.
Extraglottic airway devices. The role of newer advanced airway equipment, such as the laryngeal mask airway (LMA), in patients with poisoning or drug overdose is not known; although these devices are easier to insert than endotracheal tubes, especially in some patients with “difficult” airways, they do not provide adequate protection against pulmonary aspiration of gastric contents, and they cannot be used in patients with laryngeal edema or laryngospasm.
Along with airway problems, breathing difficulties are the major cause of morbidity and death in patients with poisoning or drug overdose. Patients may have one or more of the following complications: ventilatory failure, hypoxia, and bronchospasm.
Ventilatory failure
Assessment. Ventilatory failure has multiple causes, including failure of the ventilatory muscles, central depression of respiratory drive, and severe pneumonia or pulmonary edema. Examples of drugs and toxins that cause ventilatory failure and the causative mechanisms are listed in Table I–1.
Table I-1 Selected Drugs and Toxins Causing Ventilatory Failurea
Table I-1 Selected Drugs and Toxins Causing Ventilatory Failurea
Paralysis of ventilatory muscles
Depression of central respiratory drive
Botulinum toxin (botulism)
Antihistamines
Neuromuscular blockers
Barbiturates
Nicotine
Clonidine and other sympatholytic agents
Organophosphates and carbamates
Ethanol and alcohols
Saxitoxin (“red tide”)
Gamma-hydroxybutyrate (GHB)
Snakebite
Opioids
Strychnine and tetanus (muscle rigidity)
Phenothiazines and antipsychotic drugs
Tetrodotoxin
Sedative-hypnotics
Warfare nerve gases
Tricyclic antidepressants
Complications. Ventilatory failure is the most common cause of death in poisoned patients.
Hypoxia may result in brain damage, cardiac dysrhythmias, and cardiac arrest.
Hypercarbia results in acidosis, which may contribute to dysrhythmias, especially in patients with salicylate or tricyclic antidepressant overdoses.
Differential diagnosis. Rule out the following:
Bacterial or viral pneumonia.
Viral encephalitis or myelitis (eg, polio).
Traumatic or ischemic spinal cord or central nervous system (CNS) injury.
Tetanus, causing rigidity of chest wall muscles.
Pneumothorax.
Treatment. Obtain measurements of the arterial blood gases. Quickly estimate the adequacy of ventilation from the Pco2 level; obtundation with an elevated or rising Pco2 (eg, >60 mm Hg) indicates a need for assisted ventilation. Do not wait until the patient is apneic or until the Pco2 is above 60 mm to begin assisted ventilation.
Assist breathing manually with a bag-valve-mask device or bag-valve-endotracheal tube device until the mechanical ventilator is ready for use.
If not already accomplished, perform endotracheal intubation.
Program the ventilator for tidal volume (usually 15 mL/kg), rate (usually 12–15 breaths/min), and oxygen concentration (usually 30–35% to start). Monitor the patient’s response to the ventilator settings frequently by obtaining arterial blood gas values. Note: In salicylate-poisoned patients with severe acidosis and marked compensatory tachypnea, the ventilator should be programmed to match the patient’s high minute ventilation. Otherwise, any rise in the patient’s Pco2 and consequent fall in blood pH can dramatically increase tissue levels of salicylate, with disastrous consequences.
If the patient has some spontaneous ventilation, the machine can be set to allow the patient to breathe spontaneously with only intermittent mandatory ventilation (usually 10–12 breaths/min).
If the endotracheal tube has been placed only for airway protection, the patient can be left to breathe entirely spontaneously with blow-by oxygen mist (T-piece).
Hypoxia
Assessment. Examples of drugs or toxins causing hypoxia are listed in Table I–2. Hypoxia can be caused by the following conditions:
Table I-2 Selected Causes of Hypoxiaa
Table I-2 Selected Causes of Hypoxiaa
Inert gases
Pneumonia or noncardiogenic pulmonary edema
Carbon dioxide
Aspiration of gastric contents
Methane and propane
Aspiration of hydrocarbons
Nitrogen
Chlorine and other irritant gases
Cardiogenic pulmonary edema
Cocaine
Beta receptor antagonists
Ethchlorvynol (IV and oral)
Quinidine, procainamide, and disopyramide
Ethylene glycol
Tricyclic antidepressants
Mercury vapor
Verapamil
Metal fumes (“metal fumes fever”)
Cellular hypoxia
Nitrogen dioxide
Carbon monoxide
Opioids
Cyanide
Paraquat
Hydrogen sulfide
Phosgene
Methemoglobinemia
Salicylates
Sulfhemoglobinemia
Sedative-hypnotic drugs
Smoke inhalation
Insufficient oxygen in ambient air (eg, displacement of oxygen by inert gases).
Disruption of oxygen absorption by the lung (eg, resulting from pneumonia or pulmonary edema).
Pneumonia. The most common cause of pneumonia in overdosed patients is pulmonary aspiration of gastric contents. Pneumonia may also be caused by the IV injection of foreign material or bacteria, aspiration of petroleum distillates, or inhalation of irritant gases.
Pulmonary edema. All agents that can cause chemical pneumonia (eg, irritant gases and hydrocarbons) can also cause pulmonary edema. This usually involves an alteration of permeability in pulmonary capillaries, resulting in noncardiogenic pulmonary edema (acute respiratory distress syndrome [ARDS]). In noncardiogenic pulmonary edema, the pulmonary capillary wedge pressure (reflecting filling pressure in the left ventricle) is usually normal or low. In contrast, cardiogenic pulmonary edema caused by cardiac depressant drugs is characterized by low cardiac output with elevated pulmonary wedge pressure.
Cellular hypoxia, which may be present despite a normal arterial blood gas value.
Carbon monoxide poisoning (See Carbon monoxide) and methemoglobinemia (See Methemoglobinemia) may severely limit oxygen binding to hemoglobin (and therefore the oxygen-carrying capacity of blood) without altering the Po2 because routine blood gas determination measures dissolved oxygen in the plasma but does not measure actual oxygen content. In such cases, only the direct measurement of oxygen saturation with a co-oximeter (not its calculation from the Po2) will reveal decreased oxyhemoglobin saturation. Note: Conventional pulse oximetry gives falsely normal or inaccurate results and is not reliable. A newer pulse oximetry device (the Masimo pulse co-oximeter) can estimate carboxyhemoglobin and methemoglobin concentrations, but its accuracy and sensitivity are uncertain.
Cyanide poisoning (See Cyanide) and hydrogen sulfide poisoning (See Hydrogen Sulfide) interfere with cellular oxygen utilization, resulting in decreased oxygen uptake by the tissues, and may cause abnormally high venous oxygen saturation.
Complications. Significant or sustained hypoxia may result in brain damage and cardiac dysrhythmias.
Differential diagnosis. Rule out the following:
Erroneous sampling (eg, inadvertently measuring venous blood gases rather than arterial blood gases).
Bacterial or viral pneumonia.
Pulmonary contusion caused by trauma.
Acute myocardial infarction with pump failure.
Treatment
Correct hypoxia. Administer supplemental oxygen as indicated, based on arterial Po2. Intubation and assisted ventilation may be required.
If carbon monoxide poisoning is suspected, give 100% oxygen and consider hyperbaric oxygen (See Oxygen and Hyperbaric Oxygen).
See also treatment guides for cyanide (See Cyanide), hydrogen sulfide (See Hydrogen Sulfide), and methemoglobinemia (See Methemoglobinemia).
Treat pneumonia. Obtain sputum samples and initiate appropriate antibiotic therapy when there is evidence of infection.
Treat pulmonary edema.
Avoid excessive fluid administration. Assessment of volume status by ultrasound or pulmonary artery cannulation and wedge pressure measurements may be necessary to guide fluid therapy.
Administer supplemental oxygen to maintain a Po2 of at least 60–70 mm Hg. Endotracheal intubation and the use of positive end-expiratory pressure (PEEP) ventilation may be necessary to maintain adequate oxygenation.
Bronchospasm
Assessment. Examples of drugs and toxins that cause bronchospasm are listed in Table I–3. Bronchospasm may result from the following:
Table I-3 Selected Drugs and Toxins Causing Bronchospasm
Table I-3 Selected Drugs and Toxins Causing Bronchospasm
Beta receptor antagonists
Isocyanates
Brevetoxin
Organophosphates and other anticholinesterases
Chlorine and other irritant gases
Particulate dusts
Drugs causing allergic reactions
Smoke inhalation
Hydrocarbon aspiration
Sulfites (eg, in foods)
Direct irritant injury from the inhalation of gases or pulmonary aspiration of petroleum distillates or stomach contents.
Pharmacologic effects of toxins (eg, organophosphate or carbamate insecticides or beta-adrenergic antagonists).
Hypersensitivity or allergic reactions.
Complications. Severe bronchospasm may result in hypoxia and ventilatory failure. Exposure to high concentrations of irritant gases can lead to asthma (“reactive airway dysfunction syndrome” [RADS]).
Differential diagnosis. Rule out the following:
Asthma or other preexisting bronchospastic disorders.
Stridor caused by upper airway injury and edema (progressive airway edema may result in acute airway obstruction).
Airway obstruction by a foreign body.
Treatment
Administer supplemental oxygen. Assist ventilation and perform endotracheal intubation if needed.
Remove the patient from the source of exposure to any irritant gas or other offending agent.
Immediately discontinue any beta-adrenergic antagonist treatment.
Administer bronchodilators:
Aerosolized beta2 receptor stimulant (eg, albuterol [2.5–5 mg] in nebulizer). Repeat as needed or give 5–15 mg as a continuous nebulizer treatment over 1 hour (children: 0.3–0.5 mg/kg/h).
Aerosolized ipratropium bromide, 0.5 mg every 4–6 hours, especially if excessive cholinergic stimulation is suspected.
For reactive airways, consider inhaled or oral steroids.
For patients with bronchospasm and bronchorrhea caused by organophosphate, carbamate, or other cholinesterase inhibitor poisoning, give atropine (See Atropine and Glycopyrrolate) IV. Ipratropium bromide (see Item 4.b above) may also be helpful.
Paralysis of ventilatory muscles | Depression of central respiratory drive |
Botulinum toxin (botulism) | Antihistamines |
Neuromuscular blockers | Barbiturates |
Nicotine | Clonidine and other sympatholytic agents |
Organophosphates and carbamates | Ethanol and alcohols |
Saxitoxin (“red tide”) | Gamma-hydroxybutyrate (GHB) |
Snakebite | Opioids |
Strychnine and tetanus (muscle rigidity) | Phenothiazines and antipsychotic drugs |
Tetrodotoxin | Sedative-hypnotics |
Warfare nerve gases | Tricyclic antidepressants |
Inert gases | Pneumonia or noncardiogenic pulmonary edema |
Carbon dioxide | Aspiration of gastric contents |
Methane and propane | Aspiration of hydrocarbons |
Nitrogen | Chlorine and other irritant gases |
Cardiogenic pulmonary edema | Cocaine |
Beta receptor antagonists | Ethchlorvynol (IV and oral) |
Quinidine, procainamide, and disopyramide | Ethylene glycol |
Tricyclic antidepressants | Mercury vapor |
Verapamil | Metal fumes (“metal fumes fever”) |
Cellular hypoxia | Nitrogen dioxide |
Carbon monoxide | Opioids |
Cyanide | Paraquat |
Hydrogen sulfide | Phosgene |
Methemoglobinemia | Salicylates |
Sulfhemoglobinemia | Sedative-hypnotic drugs |
Smoke inhalation |
Beta receptor antagonists | Isocyanates |
Brevetoxin | Organophosphates and other anticholinesterases |
Chlorine and other irritant gases | Particulate dusts |
Drugs causing allergic reactions | Smoke inhalation |
Hydrocarbon aspiration | Sulfites (eg, in foods) |
General assessment and initial treatment
Check blood pressure and pulse rate and rhythm. Perform cardiopulmonary resuscitation (CPR) if there is no pulse and perform advanced cardiac life support (ACLS) for dysrhythmias and shock. Note: Some ACLS drugs may be ineffective or dangerous in patients with drug- or poison-induced cardiac disorders. For example, procainamide and other type Ia antiarrhythmic drugs are contraindicated in patients with tricyclic antidepressant or other sodium channel blocker overdose, and atropine and isoproterenol are ineffective in patients with beta receptor antagonist poisoning.
Begin continuous electrocardiographic (ECG) monitoring. Dysrhythmias may complicate a variety of drug overdoses, and all patients with potentially cardiotoxic drug poisoning should be monitored in the emergency department or an intensive care unit for at least 6 hours after the ingestion.
Secure venous access. Antecubital or forearm veins are usually easy to cannulate. Alternative sites include femoral, subclavian, internal jugular, and other central veins. Access to central veins is technically more difficult but allows measurement of the central venous pressure and placement of a pacemaker or pulmonary artery lines. Intraosseous (IO) access may also be used in urgent situations.
Draw blood for routine studies (See Diagnosis of Poisoning).
Begin IV infusion of normal saline (NS), 5% dextrose in NS (D5NS), 5% dextrose in half NS (D5W 0.45% sodium chloride), or 5% dextrose in water (D5W) at a keep-open rate; for children, use 5% dextrose in quarter NS (D5W 0.25% sodium chloride). If the patient is hypotensive (See Hypotension), NS or another isotonic crystalloid solution is preferred.
In seriously ill patients (eg, those who are hypotensive, obtunded, convulsing, or comatose), place a Foley catheter in the bladder, obtain urine for routine and toxicologic testing, and measure hourly urine output.
Bradycardia and atrioventricular (AV) block
Assessment. Examples of drugs and toxins causing bradycardia or AV block and their mechanisms are listed in Table I–4.
Table I-4 Selected Drugs and Toxins Causing Bradycardia or Atrioventricular Blocka
Table I-4 Selected Drugs and Toxins Causing Bradycardia or Atrioventricular Blocka
Cholinergic or vagotonic agents
Sympatholytic agents
Digitalis glycosides
Beta receptor antagonists
Organophosphates and carbamates
Clonidine
Physostigmine, neostigmine
Opioids
Membrane-depressant drugs
Other
Propranolol
Calcium antagonists
Encainide and flecainide
Carbamazepine
Quinidine, procainamide, and disopyramide
Lithium
Tricyclic antidepressants
Phenylpropanolamine and other alpha-adrenergic agonists
Propoxyphene
Bradycardia and AV block are common features of intoxication with calcium antagonists (See Calcium Channel Antagonists) and drugs that depress sympathetic tone or increase parasympathetic tone (eg, digoxin). These conditions may also result from severe intoxication with membrane-depressant (sodium channel–blocking) drugs (eg, tricyclic antidepressants, quinidine, and other types Ia and Ic antiarrhythmic agents).
Bradycardia or AV block may also be a reflex response (baroreceptor reflex) to hypertension induced by alpha-adrenergic agents such as phenylpropanolamine and phenylephrine.
In children, bradycardia is commonly caused by respiratory compromise and usually responds to ventilation and oxygenation.
Complications. Bradycardia and AV block frequently cause hypotension, which may progress to asystolic cardiac arrest.
Differential diagnosis. Rule out the following:
Hypothermia.
Myocardial ischemia or infarction.
Electrolyte abnormality (eg, hyperkalemia).
Metabolic disturbance (eg, hypothyroidism).
Physiologic origin, resulting from a baroreceptor response to hypertension, an intrinsically slow pulse rate (common in athletes), or an acute vasovagal reaction.
Cushing reflex (caused by severe intracranial hypertension).
Treatment. Do not treat bradycardia or AV block unless the patient is symptomatic (eg, exhibits signs of syncope or hypotension). Note: Bradycardia or even AV block may be a protective baroreceptor reflex to lower the blood pressure in a patient with severe hypertension (see Item VII below).
Maintain an open airway and assist ventilation (See Airway) if necessary. Administer supplemental oxygen.
Rewarm hypothermic patients. A sinus bradycardia of 40–50 beats/min is common when the body temperature is 32–35°C (90–95°F) and will usually return to normal with warming.
Administer atropine, 0.01–0.03 mg/kg IV (See Atropine and Glycopyrrolate). If this is not successful, use isoproterenol, 1–10 mcg/min IV (See Isoproterenol), titrated to the desired rate, or use an emergency transcutaneous or transvenous pacemaker.
Use the following specific antidotes if appropriate:
For beta receptor antagonist overdose, give glucagon (See Glucagon).
For digoxin, digitalis, or other cardiac glycoside intoxication, use Fab antibody fragments (See Digoxin-Specific Antibodies).
For tricyclic antidepressant or membrane-depressant drug overdose, administer sodium bicarbonate (See Bicarbonate, Sodium).
For calcium antagonist overdose, give calcium (See Calcium), hyperinsulin-euglycemia therapy (See Insulin), or intralipid rescue (See Lipid Emulsion).
QRS interval prolongation
Assessment. Examples of drugs and toxins causing QRS interval prolongation are listed in Table I–5.
Table I-5 Selected Drugs and Toxins Causing QRS Interval Prolongationa
Table I-5 Selected Drugs and Toxins Causing QRS Interval Prolongationa
Bupropion
Lamotrigine
Chloroquine and related agents
Phenothiazines (thioridazine)
Cocaine (high-dose)
Propoxyphene
Digitalis glycosides (complete heart block)
Propranolol
Diphenhydramine (high-dose)
Quinidine, procainamide, and disopyramide
Encainide and flecainide
Tricyclic antidepressants
Hyperkalemia
Venlafaxine
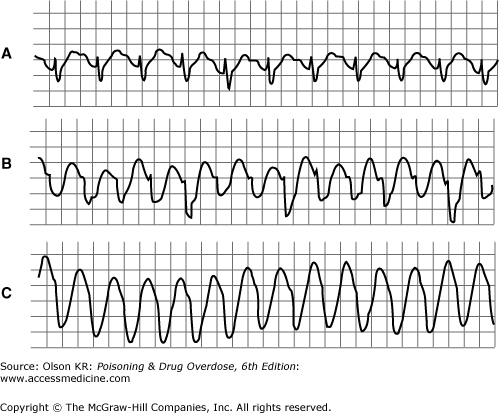
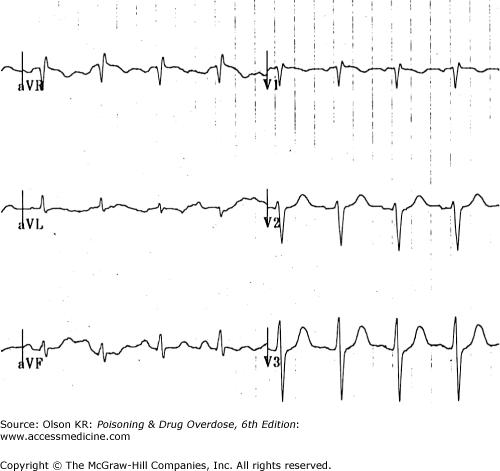
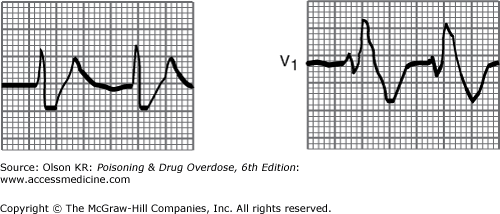
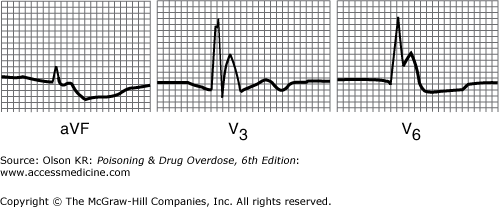
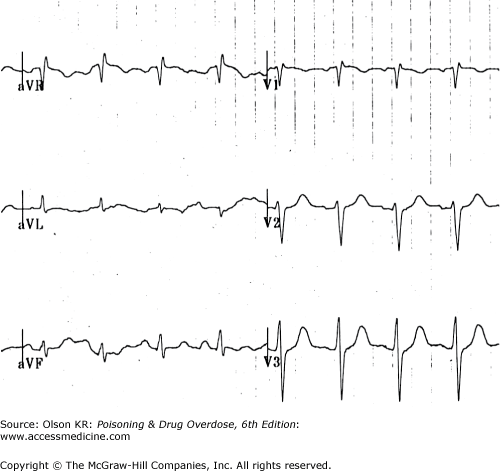
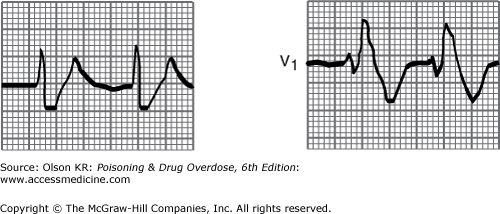
QRS interval prolongation of greater than 0.12 second in the limb leads (Figure I–3) strongly indicates serious poisoning by tricyclic antidepressants (See Antidepressants, Tricyclic) or other membrane-depressant drugs (eg, quinidine (See Quinidine and Other Type IA Antiarrhythmic Drugs), flecainide [See Antiarrhythmic Drugs], chloroquine [See Chloroquine and Other Aminoquinolines], and propranolol [See Beta-Adrenergic Blockers]). Rightward axis deviation of the terminal 40 milliseconds of the ECG, which is easily recognized as a late R wave in the aVR lead, may precede QRS widening in patients with tricyclic antidepressant intoxication (Figure I–4).
QRS interval prolongation may also result from a ventricular escape rhythm in a patient with complete heart block (eg, from digitalis, calcium antagonist poisoning, or intrinsic cardiac disease).
Complications. QRS interval prolongation in patients with tricyclic antidepressant or similar drug poisoning is often accompanied by hypotension, AV block, and seizures.
Differential diagnosis. Rule out the following:
Intrinsic conduction system disease (bundle branch block or complete heart block) caused by coronary artery disease. Check an old ECG if available.
Brugada syndrome.
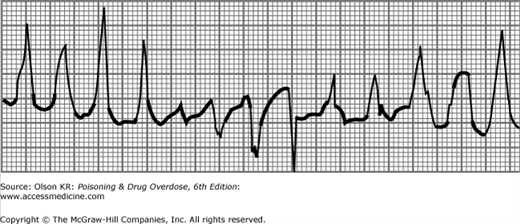
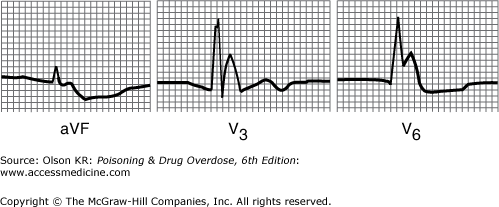
Hyperkalemia with critical cardiac toxicity may appear as a “sine wave” pattern with markedly wide QRS complexes. These are usually preceded by peaked T waves (Figure I–5).
Hypothermia with a core temperature of less than 32°C (90°F) often causes an extraterminal QRS deflection (J wave or Osborne wave), resulting in a widened QRS appearance (Figure I–6).
Treatment
Maintain the airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Treat hyperkalemia (See Diagnosis of Poisoning) and hypothermia (See Altered Mental Status) if they occur.
Treat AV block with atropine (See Atropine and Glycopyrrolate), isoproterenol (See Isoproterenol), and a pacemaker if necessary.
For tricyclic antidepressant or other sodium channel–blocking drug overdose, give sodium bicarbonate, 1- to 2-mEq/kg IV bolus (See Bicarbonate, Sodium); repeat as needed.
Tachycardia
Assessment. Examples of drugs and toxins causing tachycardia and their mechanisms are listed in Table I–6.
Table I-6 Selected Drugs and Toxins Causing Tachycardiaa
Table I-6 Selected Drugs and Toxins Causing Tachycardiaa
Sympathomimetic agents
Anticholinergic agents
Amphetamines and derivatives
Amanita muscaria mushrooms
Caffeine
Antihistamines
Cocaine
Atropine and other anticholinergics
Ephedrine and pseudoephedrine
Phenothiazines
Phencyclidine (PCP)
Plants (many [See Plants])
Theophylline
Tricyclic antidepressants
Agents causing cellular hypoxia
Other
Carbon monoxide
Ethanol or sedative-hypnotic drug withdrawal
Cyanide
Vasodilators (reflex tachycardia)
Hydrogen sulfide
Thyroid hormone
Oxidizing agents (methemoglobinemia)
Sinus tachycardia and supraventricular tachycardia are often caused by excessive sympathetic stimulation or inhibition of parasympathetic tone. Sinus tachycardia may also be a reflex response to hypotension or hypoxia.
Sinus tachycardia and supraventricular tachycardia accompanied by QRS interval prolongation (eg, with tricyclic antidepressant poisoning) may have the appearance of ventricular tachycardia (see Figure I–3).
Complications. Simple sinus tachycardia (heart rate <140 beats/min) is rarely of hemodynamic consequence; children and healthy adults easily tolerate rates of up to 160–180 beats/min. However, sustained rapid rates may result in hypotension, chest pain, myocardial ischemia, or syncope.
Differential diagnosis. Rule out the following:
Occult blood loss (eg, from gastrointestinal bleeding or trauma).
Fluid loss (eg, from gastritis or gastroenteritis).
Hypoxia.
Fever and infection.
Myocardial infarction.
Anxiety.
Intrinsic conduction system disease (eg, Wolff-Parkinson-White syndrome).
Treatment. If tachycardia is not associated with hypotension or chest pain, observation and sedation (especially for stimulant intoxication) are usually adequate.
For sympathomimetic-induced tachycardia, give esmolol, 0.025–0.1 mg/kg/min IV (See Esmolol). Note: If tachycardia is accompanied by hypertension, add a vasodilator (see Section VII.D.2 below).
Anticholinergic-induced tachycardia may respond to physostigmine (See Physostigmine and Neostigmine) or neostigmine, but tachycardia alone is rarely an indication for use of these drugs. Moreover, in patients with tricyclic antidepressant overdose, additive depression of conduction by these drugs may result in severe bradycardia, heart block, or asystole.
Ventricular dysrhythmias
Assessment. Examples of drugs and toxins causing ventricular dysrhythmias are listed in Table I–7.
Table I-7 Selected Drugs and Toxins Causing Ventricular Arrhythmiasa
Table I-7 Selected Drugs and Toxins Causing Ventricular Arrhythmiasa
Ventricular tachycardia or fibrillation
Amphetamines and other sympathomimetic agents
Digitalis glycosides
Aromatic hydrocarbon solvents
Fluoride
Caffeine
Phenothiazines
Chloral hydrate
Theophylline
Chlorinated or fluorinated hydrocarbon solvents
Tricyclic antidepressants
Cocaine
QT prolongation with well-documented risk for torsade de pointesb
Amiodarone
Ibutilide
Arsenic trioxide
Levomethadyl
Astemizole
Mesoridazine
Bepridil
Methadone
Chloroquine
Pentamidine
Chlorpromazine
Pimozide
Cisapride
Probucol
Clarithromycin
Procainamide
Disopyramide
Organophosphate insecticides
Dofetilide
Quinidine
Domperidone
Sotalol
Droperidol
Sparfloxacin
Erythromycin
Terfenadine
Halofantrine
Thallium
Haloperidol
Thioridazine
Ventricular irritability is commonly associated with excessive sympathetic stimulation (eg, from cocaine or amphetamines). Patients intoxicated by chlorinated, fluorinated, or other hydrocarbons may have heightened myocardial sensitivity to the arrhythmogenic effects of catecholamines.
Ventricular tachycardia may also be a manifestation of intoxication by a tricyclic antidepressant or another sodium channel–blocking drug, although with these drugs true ventricular tachycardia may be difficult to distinguish from sinus or supraventricular tachycardia accompanied by QRS interval prolongation (see Figure I–3).
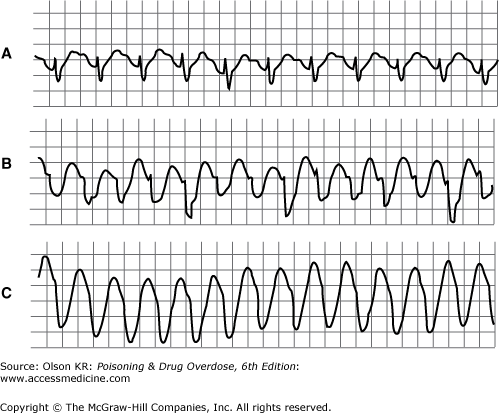
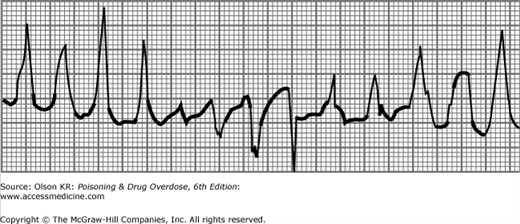
Agents that cause QT interval prolongation (QTc >0.43 seconds in men, >0.45 seconds in women) may produce “atypical” ventricular tachycardia (torsade de pointes). Torsade de pointes is a polymorphous ventricular tachycardia in which the axis appears to rotate continuously (Figure I–7). Torsade de pointes may also be caused by hypokalemia, hypocalcemia, or hypomagnesemia.
Complications. Ventricular tachycardia in patients with a pulse may be associated with hypotension or may deteriorate into pulseless ventricular tachycardia or ventricular fibrillation.
Differential diagnosis. Rule out the following possible causes of ventricular premature beats, ventricular tachycardia, or ventricular fibrillation:
Hypoxemia.
Hypokalemia.
Metabolic acidosis.
Myocardial ischemia or infarction.
Electrolyte disturbances (eg, hypocalcemia or hypomagnesemia) or congenital disorders that may cause QT prolongation and torsade de pointes.
Brugada syndrome.
Treatment. Perform CPR if necessary and follow standard ACLS guidelines for the management of dysrhythmias, with the exception that type Ia antiarrhythmic drugs should not be used, especially if tricyclic antidepressant or sodium channel–blocking drug overdose is suspected.
Maintain an open airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Correct acid-base and electrolyte disturbances.
For ventricular fibrillation, immediately apply direct current countershock at 3–5 J/kg. Repeat twice if no response. Continue CPR if the patient is still without a pulse and administer epinephrine, repeated countershocks, amiodarone, and/or lidocaine as recommended in advanced cardiac life support (ACLS) guidelines.
For ventricular tachycardia in patients without a pulse, immediately give a precordial thump or apply synchronized direct current countershock at 1–3 J/kg. If this is not successful, begin CPR and apply countershock at 3–5 J/kg; administer amiodarone and/or lidocaine and repeated countershocks as recommended in ACLS guidelines.
For ventricular tachycardia in patients with a pulse, use lidocaine, 1–3 mg/kg IV (See Lidocaine), or amiodarone, 300 mg IV or 5 mg/kg in children. Do not use procainamide or other type Ia antiarrhythmic agents. For suspected myocardial sensitivity caused by chloral hydrate or halogenated or aromatic hydrocarbons, use esmolol, 0.025–0.1 mg/kg/min IV (See Esmolol), or propranolol, 0.5–3 mg IV (See Propranolol).
For tricyclic antidepressant or other sodium channel–blocking drug overdose, administer sodium bicarbonate, 1–2 mEq/kg IV (See Bicarbonate, Sodium) in repeated boluses until the QRS interval narrows or the serum pH exceeds 7.7.
For “atypical” or polymorphic ventricular tachycardia (torsade de pointes), do the following:
Administer IV magnesium sulfate, 1–2 g in adults, over 20–30 minutes (See Magnesium).
Use overdrive pacing or isoproterenol, 1–10 mcg/min IV (See Isoproterenol), to increase the heart rate (this makes repolarization more homogeneous and abolishes the dysrhythmia).
Hypotension
Assessment. Examples of drugs and toxins causing hypotension and their mechanisms are listed in Table I–8.
Table I-8 Selected Drugs and Toxins Causing Hypotensiona
Table I-8 Selected Drugs and Toxins Causing Hypotensiona
HYPOTENSION WITH RELATIVE BRADYCARDIA
Sympatholytic agents
Beta receptor antagonists
Bretylium
Clonidine and methyldopa
Hypothermia
Opioids
Reserpine
Tetrahydrozoline and oxymetazoline
Membrane-depressant drugs
Encainide and flecainide
Quinidine, procainamide, and disopyramide
Propoxyphene
Propranolol
Tricyclic antidepressants
Others
Barbiturates
Calcium antagonists (verapamil, diltiazem)
Fluoride
Organophosphates and carbamates
Sedative-hypnotic agents
Tilmicosin
HYPOTENSION WITH TACHYCARDIA
Fluid loss or third spacing
Amatoxin-containing mushrooms
Arsenic
Colchicine
Copper sulfate
Hyperthermia
Iron
Rattlesnake envenomation
Sedative-hypnotic agents
Peripheral venous or arteriolar dilation
Alpha antagonists (doxazosin, prazosin, terazosin)
Beta2 receptor agonists (eg, albuterol)
Caffeine
Calcium antagonists (nifedipine, amlodipine, nicardipine)
Hydralazine
Hyperthermia
Minoxidil
Nitrites
Sodium nitroprusside
Phenothiazines
Quetiapine
Theophylline
Tricyclic antidepressants
Physiologic derangements resulting in hypotension include volume loss because of vomiting, diarrhea, or bleeding; apparent volume depletion caused by venodilation, arteriolar dilation, depression of cardiac contractility, and dysrhythmias that interfere with cardiac output; and hypothermia.
Volume loss, venodilation, and arteriolar dilation are likely to result in hypotension with reflex tachycardia. In contrast, hypotension accompanied by bradycardia should suggest intoxication by sympatholytic agents, membrane-depressant drugs, calcium antagonists, or cardiac glycosides, or the presence of hypothermia.
Complications. Severe or prolonged hypotension can cause acute renal tubular necrosis, brain damage, and cardiac ischemia. Metabolic acidosis is a common finding.
Differential diagnosis. Rule out the following:
Hypothermia, which results in a decreased metabolic rate and lowered blood pressure demands.
Hyperthermia, which causes arteriolar dilation and venodilation and direct myocardial depression.
Fluid loss caused by gastroenteritis.
Blood loss (eg, from trauma or gastrointestinal bleeding).
Myocardial infarction.
Sepsis.
Spinal cord injury.
Treatment. Fortunately, hypotension usually responds readily to empiric therapy with IV fluids and low doses of vasoactive drugs (eg, dopamine, norepinephrine). When hypotension does not resolve after simple measures, a systematic approach should be followed to determine the cause of hypotension and select the appropriate treatment.
Maintain an open airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Treat cardiac dysrhythmias that may contribute to hypotension (heart rate <40–50 beats/min or >180–200 beats/min [See Circulation]).
Hypotension associated with hypothermia often will not be relieved with routine fluid therapy, but the pressure will normalize rapidly on rewarming of the patient. A systolic blood pressure of 80–90 mm Hg is expected when the body temperature is 32°C (90°F).
Give an IV fluid challenge with NS, 10–20 mL/kg, or another crystalloid solution.
Administer dopamine, 5–15 mcg/kg/min (See Dopamine). Note that dopamine may be ineffective in some patients with depleted neuronal stores of catecholamines (eg, from disulfiram [See Disulfiram], reserpine, or tricyclic antidepressant [See Antidepressants, Tricyclic] overdose) or in patients in whom alpha-adrenergic receptors may be blocked (tricyclic antidepressants, phenothiazines). In such cases, norepinephrine, 0.1 mcg/kg/min IV (See Norepinephrine), or phenylephrine (See Phenylephrine) may be more effective.
Consider specific antidotes:
Sodium bicarbonate (See Bicarbonate, Sodium) for tricyclic antidepressant or other sodium channel–blocking drug overdose.
Glucagon (See Glucagon) for beta receptor antagonist overdose.
Calcium (See Calcium) for calcium antagonist overdose.
Propranolol (See Propranolol) or esmolol (See Esmolol) for theophylline, caffeine, or metaproterenol or other beta agonist overdose.
Other treatments:
Severe hypotension due to calcium antagonist or beta blocker poisoning may respond to hyperinsulin-euglycemia therapy (See Insulin).
Intralipid rescue (See Lipid Emulsion) may be useful for severe cardiotoxicity due to lipid-soluble drugs (eg, bupivacaine, verapamil, bupropion).
If adrenal insufficiency is suspected, administer corticosteroids (eg, hydrocortisone, 100 mg IV every 8 hours).
If empiric measures to restore the blood pressure are unsuccessful, assess volume status and cardiac contractility with bedside ultrasound, or insert a central venous pressure (CVP) monitor or pulmonary artery catheter to determine whether further IV fluids are needed and to measure the cardiac output (CO) and calculate the systemic vascular resistance (SVR):

Select further therapy on the basis of the following:
If the central venous pressure or pulmonary artery wedge pressure remains low, give more IV fluids.
If the cardiac output is low, give more dopamine or dobutamine.
If the systemic vascular resistance is low, administer norepinephrine, 4–8 mcg/min (See Norepinephrine), or phenylephrine (See Phenylephrine).
Hypertension
Assessment. Hypertension is frequently overlooked in drug-intoxicated patients and often goes untreated. Many young people have normal blood pressures in the range of 90/60 to 100/70 mm Hg; in such a person, an abrupt elevation to 170/100 mm Hg is much more significant (and potentially catastrophic) than the same blood pressure elevation in an older person with chronic hypertension. Examples of drugs and toxins causing hypertension are listed in Table I–9. Hypertension may be caused by a variety of mechanisms:
Table I-9 Selected Drugs and Toxins Causing Hypertensiona
Table I-9 Selected Drugs and Toxins Causing Hypertensiona
HYPERTENSION WITH TACHYCARDIA
Generalized sympathomimetic agents
Anticholinergic agentsb
Amphetamines and derivatives
Antihistamines
Cocaine
Atropine and other anticholinergics
Ephedrine and pseudoephedrine
Tricyclic antidepressants
Epinephrine
Others
Levodopa
Ethanol and sedative-hypnotic drug withdrawal
LSD (lysergic acid diethylamide)
Nicotine (early stage)
Marijuana
Organophosphates (early stage)
Monoamine oxidase inhibitors
HYPERTENSION WITH BRADYCARDIA OR ATRIOVENTRICULAR BLOCK
Clonidine, tetrahydrozoline, and oxymetazoline c
Norepinephrine
Ergot derivatives
Phenylephrine
Methoxamine
Phenylpropanolamine
Amphetamines and other related drugs cause hypertension and tachycardia through generalized sympathetic stimulation.
Selective alpha-adrenergic agents cause hypertension with reflex (baroreceptor-mediated) bradycardia or even AV block.
Anticholinergic agents cause mild hypertension with tachycardia.
Substances that stimulate nicotinic cholinergic receptors (eg, organophosphates) may initially cause tachycardia and hypertension, followed later by bradycardia and hypotension.
Withdrawal from sedative-hypnotic drugs, ethanol, opioids, or clonidine can cause hypertension and tachycardia.
Complications. Severe hypertension can result in intracranial hemorrhage, aortic dissection, myocardial infarction, and congestive heart failure.
Differential diagnosis. Rule out the following:
Idiopathic hypertension (which is common in the general population). However, without a prior history of hypertension, it should not be initially assumed to be the cause of the elevated blood pressure.
Pheochromocytoma or other paraganglionic tumors that secrete epinephrine, norepinephrine, or both are rare but potentially lethal. They typically cause paroxysmal attacks of hypertension, headache, perspiration, and palpitations.
Increased intracranial pressure caused by spontaneous hemorrhage, trauma, or other causes. This may result in hypertension with reflex bradycardia (Cushing reflex).
Treatment. Rapid lowering of the blood pressure is desirable as long as it does not result in hypotension, which can potentially cause an ischemic cerebral infarction in older patients with cerebrovascular disease. For a patient with chronic hypertension, lowering the diastolic pressure to 100 mm Hg is acceptable. However, for a young person whose normal diastolic blood pressure is 60 mm Hg, the diastolic pressure should be lowered to 80 mm Hg.
For hypertension with little or no tachycardia, use phentolamine, 0.02–0.1 mg/kg IV (See Phentolamine), or nitroprusside, 2–10 mcg/kg/min IV (See Nitroprusside).
For hypertension with tachycardia, add to the treatment in Item 1 above propranolol, 0.02–0.1 mg/kg IV (See Propranolol), or esmolol, 0.025–0.1 mg/kg/min IV (See Esmolol), or labetalol, 0.2–0.3 mg/kg IV (See Hydroxocobalamin). Caution: Do not use propranolol or esmolol without a vasodilator to treat hypertensive crisis; beta receptor antagonists may paradoxically worsen hypertension because any alpha-mediated vasoconstriction is unopposed when beta2-mediated vasodilation is blocked.
If hypertension is accompanied by a focally abnormal neurologic examination (eg, hemiparesis), perform computed tomography (CT) as quickly as possible. In a patient with a cerebrovascular accident, hypertension should generally not be treated unless specific complications of the elevated pressure (eg, heart failure or cardiac ischemia) are present. Consult a neurologist or neurosurgeon.
Cholinergic or vagotonic agents | Sympatholytic agents |
Digitalis glycosides | Beta receptor antagonists |
Organophosphates and carbamates | Clonidine |
Physostigmine, neostigmine | Opioids |
Membrane-depressant drugs | Other |
Propranolol | Calcium antagonists |
Encainide and flecainide | Carbamazepine |
Quinidine, procainamide, and disopyramide | Lithium |
Tricyclic antidepressants | Phenylpropanolamine and other alpha-adrenergic agonists |
Propoxyphene |
Bupropion | Lamotrigine |
Chloroquine and related agents | Phenothiazines (thioridazine) |
Cocaine (high-dose) | Propoxyphene |
Digitalis glycosides (complete heart block) | Propranolol |
Diphenhydramine (high-dose) | Quinidine, procainamide, and disopyramide |
Encainide and flecainide | Tricyclic antidepressants |
Hyperkalemia | Venlafaxine |
Figure I–3.
Widened QRS interval caused by tricyclic antidepressant overdose. A: Delayed intraventricular conduction results in prolonged QRS interval (0.18 s). B and C: Supraventricular tachycardia with progressive widening of QRS complexes mimics ventricular tachycardia. (Modified and reproduced, with permission, from Benowitz NL, Goldschlager N: Cardiac disturbances in the toxicologic patient. In: Haddad LM, Winchester JF [editors]: Clinical Management of Poisoning and Drug Overdose, p 71. WB Saunders, 1983.)
Figure I–5.
Electrocardiogram of a patient with hyperkalemia. (Modified and reproduced, with permission, from Goldschlager N, Goldman MJ: Effect of drugs and electrolytes on the electrocardiogram. In: Goldschlager N, Goldman MJ [editors]: Electrocardiography: Essentials of Interpretation, p 199. Appleton & Lange, 1984.)
Figure I–6.
Electrocardiogram of a patient with hypothermia, showing prominent J waves. (Modified and reproduced, with permission, from Goldschlager N, Goldman MJ: Miscellaneous abnormal electrocardiogram patterns. In: Goldschlager N, Goldman MJ [editors]: Electrocardiography: Essentials of Interpretation, p 227. Appleton & Lange, 1984.)
Sympathomimetic agents | Anticholinergic agents |
Amphetamines and derivatives | Amanita muscaria mushrooms |
Caffeine | Antihistamines |
Cocaine | Atropine and other anticholinergics |
Ephedrine and pseudoephedrine | Phenothiazines |
Phencyclidine (PCP) | Plants (many [See Plants]) |
Theophylline | Tricyclic antidepressants |
Agents causing cellular hypoxia | Other |
Carbon monoxide | Ethanol or sedative-hypnotic drug withdrawal |
Cyanide | Vasodilators (reflex tachycardia) |
Hydrogen sulfide | Thyroid hormone |
Oxidizing agents (methemoglobinemia) |
Ventricular tachycardia or fibrillation | |
Amphetamines and other sympathomimetic agents | Digitalis glycosides |
Aromatic hydrocarbon solvents | Fluoride |
Caffeine | Phenothiazines |
Chloral hydrate | Theophylline |
Chlorinated or fluorinated hydrocarbon solvents | Tricyclic antidepressants |
Cocaine | |
QT prolongation with well-documented risk for torsade de pointesb | |
Amiodarone | Ibutilide |
Arsenic trioxide | Levomethadyl |
Astemizole | Mesoridazine |
Bepridil | Methadone |
Chloroquine | Pentamidine |
Chlorpromazine | Pimozide |
Cisapride | Probucol |
Clarithromycin | Procainamide |
Disopyramide | Organophosphate insecticides |
Dofetilide | Quinidine |
Domperidone | Sotalol |
Droperidol | Sparfloxacin |
Erythromycin | Terfenadine |
Halofantrine | Thallium |
Haloperidol | Thioridazine |
Figure I–7.
Polymorphic ventricular tachycardia (torsade de pointes). (Modified and reproduced, with permission, from Goldschlager N, Goldman MJ: Effect of drugs and electrolytes on the electrocardiogram. In: Goldschlager N, Goldman MJ [editors]: Electrocardiography: Essentials of Interpretation, p 197. Appleton & Lange, 1984.)
HYPOTENSION WITH RELATIVE BRADYCARDIA |
Sympatholytic agents |
Beta receptor antagonists |
Bretylium |
Clonidine and methyldopa |
Hypothermia |
Opioids |
Reserpine |
Tetrahydrozoline and oxymetazoline |
Membrane-depressant drugs |
Encainide and flecainide |
Quinidine, procainamide, and disopyramide |
Propoxyphene |
Propranolol |
Tricyclic antidepressants |
Others |
Barbiturates |
Calcium antagonists (verapamil, diltiazem) |
Fluoride |
Organophosphates and carbamates |
Sedative-hypnotic agents |
Tilmicosin |
HYPOTENSION WITH TACHYCARDIA |
Fluid loss or third spacing |
Amatoxin-containing mushrooms |
Arsenic |
Colchicine |
Copper sulfate |
Hyperthermia |
Iron |
Rattlesnake envenomation |
Sedative-hypnotic agents |
Peripheral venous or arteriolar dilation |
Alpha antagonists (doxazosin, prazosin, terazosin) |
Beta2 receptor agonists (eg, albuterol) |
Caffeine |
Calcium antagonists (nifedipine, amlodipine, nicardipine) |
Hydralazine |
Hyperthermia |
Minoxidil |
Nitrites |
Sodium nitroprusside |
Phenothiazines |
Quetiapine |
Theophylline |
Tricyclic antidepressants |
HYPERTENSION WITH TACHYCARDIA | |
Generalized sympathomimetic agents | Anticholinergic agentsb |
Amphetamines and derivatives | Antihistamines |
Cocaine | Atropine and other anticholinergics |
Ephedrine and pseudoephedrine | Tricyclic antidepressants |
Epinephrine | Others |
Levodopa | Ethanol and sedative-hypnotic drug withdrawal |
LSD (lysergic acid diethylamide) | Nicotine (early stage) |
Marijuana | Organophosphates (early stage) |
Monoamine oxidase inhibitors | |
HYPERTENSION WITH BRADYCARDIA OR ATRIOVENTRICULAR BLOCK | |
Clonidine, tetrahydrozoline, and oxymetazoline c | Norepinephrine |
Ergot derivatives | Phenylephrine |
Methoxamine | Phenylpropanolamine |
Coma and stupor
Assessment. A decreased level of consciousness is the most common serious complication of drug overdose or poisoning. Examples of drugs and toxins that cause coma are listed in Table I–10.
Table I-10 Selected Drugs and Toxins Causing Coma or Stupora
Table I-10 Selected Drugs and Toxins Causing Coma or Stupora
General central nervous system depressants
Cellular hypoxia
Anticholinergics
Carbon monoxide
Antihistamines
Cyanide
Barbiturates
Hydrogen sulfide
Benzodiazepines
Methemoglobinemia
Carbamazepine
Sodium azide
Ethanol and other alcohols
Other or unknown mechanisms
GHB (gamma hydroxybutyrate)
Bromide
Phenothiazines
Diquat
Sedative-hypnotic agents
Disulfiram
Tricyclic antidepressants
Hypoglycemic agents
Valproic acid
Lithium
Sympatholytic agents
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Clonidine, tetrahydrozoline, and oxymetazoline
Phencyclidine (PCP)
Methyldopa
Salicylates
Opioids
Coma is most often a result of global depression of the brain’s reticular activating system, caused by anticholinergic agents, sympatholytic drugs, generalized CNS depressants, or toxins that result in cellular hypoxia.
Coma sometimes represents a postictal phenomenon after a drug- or toxin-induced seizure.
Coma may also be caused by brain injury associated with infarction or intracranial bleeding. Brain injury is suggested by the presence of focal neurologic deficits and is confirmed by CT.
Complications. Coma frequently is accompanied by respiratory depression, which is a major cause of death. Other conditions that may accompany or complicate coma include hypotension (See Hypotension), hypothermia (See Hypothermia), hyperthermia (See Hyperthermia), and rhabdomyolysis (See Rhabdomyolysis).
Differential diagnosis. Rule out the following:
Head trauma or other causes of intracranial bleeding.
Abnormal levels of blood glucose, sodium, or other electrolytes. Hypoglycemia is a common cause of altered mental status.
Hypoxia.
Hypothyroidism.
Liver or renal failure.
Environmental hyperthermia or hypothermia.
Serious infections such as encephalitis and meningitis.
Treatment
Maintain the airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Consider administration of dextrose, thiamine, naloxone, and possibly flumazenil.
Dextrose. All patients with depressed consciousness should receive concentrated dextrose unless hypoglycemia is ruled out with an immediate bedside glucose determination. Use a secure vein and avoid extravasation; concentrated dextrose is highly irritating to tissues. Initial doses include the following:
Adults: 50% dextrose, 50 mL (25 g) IV.
Children: 25% dextrose, 2 mL/kg IV.
Thiamine. Thiamine is given to prevent or treat Wernicke syndrome resulting from thiamine deficiency in alcoholic patients and others with suspected vitamin deficiencies. It is not given routinely to children. Give thiamine, 100 mg, in the IV solution or IM (See Thiamine (Thiamin, Vitamin B1)).
Naloxone. All patients with respiratory depression should receive naloxone (See Naloxone and Nalmefene); if a patient is already intubated and is being artificially ventilated, naloxone is not immediately necessary and can be considered a diagnostic rather than a therapeutic drug. Caution: Although naloxone has no CNS depressant activity of its own and normally can be given safely in large doses, it may precipitate abrupt opioid withdrawal. If an amphetamine or cocaine has been injected or consumed along with heroin, reversal of the opioid-induced sedation may unmask stimulant-mediated hypertension, tachycardia, or psychosis. In addition, acute pulmonary edema is sometimes temporally associated with abrupt naloxone reversal of opioid intoxication.
Give naloxone, 0.2–0.4 mg IV (may also be given IM or through an intraosseous line).
If there is no response within 1–2 minutes, give naloxone, 2 mg IV.
If there is still no response and opioid overdose is highly suspected by the history or clinical presentation (pinpoint pupils, apnea, or hypotension), give naloxone, up to 10–20 mg IV.
Consider flumazenil if benzodiazepines are the only suspected cause of coma and there are no contraindications (See Flumazenil). Caution: The use of flumazenil can precipitate seizures in patients who are dependent on benzodiazepines or who have co-ingested a convulsant drug or poison.
Normalize the body temperature (see “Hypothermia” and “Hyperthermia”).
If there is any question of CNS trauma or cerebrovascular accident, perform a CT scan of the head.
If meningitis or encephalitis is suspected, perform a lumbar puncture and treat with appropriate antibiotics.
Hypothermia
Assessment. Hypothermia may mimic or complicate drug overdose and should be suspected in every comatose patient. Examples of drugs and toxins that cause hypothermia are listed in Table I–11.
Hypothermia is usually caused by exposure to low ambient temperatures in a patient with blunted thermoregulatory response mechanisms. Drugs and toxins may induce hypothermia by causing vasodilation, inhibiting the shivering response, decreasing metabolic activity, or causing loss of consciousness in a cold environment.
A patient whose temperature is lower than 30°C (86°F) may appear to be dead, with a barely detectable pulse or blood pressure and without reflexes. The ECG may reveal an abnormal terminal deflection (J wave or Osborne wave [see Figure I–6]).
Complications. Because there is a generalized reduction of metabolic activity and less demand for blood flow, hypothermia is commonly accompanied by hypotension and bradycardia.
Mild hypotension (systolic blood pressure of 70–90 mm Hg) in a patient with hypothermia should not be treated aggressively; excessive IV fluids may cause fluid overload and further lowering of the temperature.
Severe hypothermia (temperature <28–30°C) may cause intractable ventricular fibrillation and cardiac arrest. This may occur abruptly, such as when the patient is moved or rewarmed too quickly or when CPR is performed.
Differential diagnosis. Rule out the following:
Sepsis.
Hypoglycemia.
Hypothyroidism.
Adrenal insufficiency.
Thiamine deficiency.
Treatment
Maintain the airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Because the pulse rate may be profoundly slow and weak, perform careful cardiac evaluation before assuming that the patient is in cardiac arrest. Do not treat bradycardia; it will resolve with rewarming.
Unless the patient is in cardiac arrest (asystole or ventricular fibrillation), rewarm slowly (with blankets, warmed IV fluids, and inhalation of warmed mist) to prevent rewarming dysrhythmias.
For patients in cardiac arrest, usual antiarrhythmic agents and direct current countershock are frequently ineffective until the core temperature is above 30–32°C (86–90°F). Perform CPR and initiate active internal rewarming (eg, pleural, gastric or peritoneal lavage with warmed fluids; extracorporeal bypass). For refractory ventricular fibrillation, bretylium, 5–10 mg/kg IV, was recommended in the past, but the drug is no longer available in the United States.
Open cardiac massage, with direct warm irrigation of the ventricle, or a partial cardiopulmonary bypass may be necessary in hypothermic patients in cardiac arrest who are unresponsive to the above treatment.
If the patient is hypoglycemic, give dextrose and thiamine (See Altered Mental Status).
If adrenal insufficiency is suspected, draw blood for a serum cortisol level and administer 100 mg of hydrocortisone IV.
Hyperthermia
Assessment. Hyperthermia (temperature >40°C or 104°F) may be a catastrophic complication of intoxication by a variety of drugs and toxins (Table I–12). It can be caused by excessive heat generation resulting from sustained seizures, rigidity, or other muscular hyperactivity; an increased metabolic rate; impaired dissipation of heat secondary to impaired sweating (eg, anticholinergic agents); or hypothalamic disorders.
Table I-12 Selected Drugs and Toxins Associated with Hyperthermiaa
Table I-12 Selected Drugs and Toxins Associated with Hyperthermiaa
Excessive muscular hyperactivity, rigidity, or seizures
Impaired heat dissipation or disrupted thermoregulation
Amoxapine
Amoxapine
Amphetamines and derivatives (including MDMA)
Anticholinergic agents
Cocaine
Antihistamines
Lithium
Phenothiazines and other antipsychotic agents
LSD (lysergic acid diethylamide)
Tricyclic antidepressants
Maprotiline
Other
Monoamine oxidase inhibitors
Exertional heatstroke
Phencyclidine (PCP)
Malignant hyperthermia
Tricyclic antidepressants
Metal fume fever
Increased metabolic rate
Neuroleptic malignant syndrome (NMS)
Dinitrophenol and pentachlorophenol
Serotonin syndrome
Salicylates
Withdrawal from ethanol or sedative-hypnotic drugs
Thyroid hormone
Neuroleptic malignant syndrome (NMS) is a hyperthermic disorder seen in some patients taking antipsychotic agents and is characterized by hyperthermia, muscle rigidity (often so severe as to be called “lead pipe” rigidity), metabolic acidosis, and confusion.
Malignant hyperthermia is an inherited disorder that causes severe hyperthermia, metabolic acidosis, and rigidity after the administration of certain anesthetic agents (most commonly succinylcholine and inhaled anesthetics).
Serotonin syndrome occurs primarily in patients taking monoamine oxidase (MAO) inhibitors (See Monoamine Oxidase Inhibitors) who also take serotonin-enhancing drugs such as meperidine, fluoxetine, or other selective serotonin reuptake inhibitors (SSRIs; see “Antidepressants”) and is characterized by irritability, muscle rigidity and myoclonus (especially of the lower extremities), diaphoresis, autonomic instability, and hyperthermia. It may also occur in people who have taken an overdose or a combination of SSRIs, even without the concurrent use of MAO inhibitors.
Complications. Untreated, severe hyperthermia is likely to result in hypotension, rhabdomyolysis, coagulopathy, cardiac and renal failure, brain injury, and death. Survivors often have permanent neurologic sequelae.
Differential diagnosis. Rule out the following:
Sedative-hypnotic drug or ethanol withdrawal (delirium tremens).
Exertional or environmental heat stroke.
Thyrotoxicosis.
Meningitis or encephalitis.
Other serious infections.
Treatment. Immediate rapid cooling is essential to prevent death or serious brain damage.
Maintain the airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Administer glucose-containing IV fluids and give a concentrated glucose bolus (See Glucose) if the patient is hypoglycemic.
Rapidly gain control of seizures (see below), agitation (See Agitation, delirium, or psychosis), or muscular rigidity (See Other Complications).
Begin external cooling with tepid (lukewarm) sponging and fanning. This evaporative method is the most efficient method of cooling.
Shivering often occurs with rapid external cooling, and it may generate even more heat. Some physicians recommend chlorpromazine to abolish shivering, but this agent can lower the seizure threshold, inhibit sweating, and cause hypotension. It is preferable to use a benzodiazepine such as diazepam, 0.1–0.2 mg/kg IV, or lorazepam, 0.05–0.1 mg/kg IV, or midazolam, 0.05–0.1 mg/kg IV or IM (See Benzodiazepines (Diazepam, Lorazepam, and Midazolam)), or to use neuromuscular paralysis (see below).
The most rapidly effective and reliable means of lowering the temperature is neuromuscular paralysis. Administer a nondepolarizing agent (See Neuromuscular Blockers) such as vecuronium, 0.1 mg/kg IV. Caution: The patient will stop breathing; be prepared to ventilate and intubate endotracheally.
Malignant hyperthermia. If muscle rigidity persists despite administration of neuromuscular blockers, a defect at the muscle cell level (ie, malignant hyperthermia) should be suspected. Give dantrolene, 1–10 mg/kg IV (See Cyproheptadine).
Neuroleptic malignant syndrome (NMS). Consider bromocriptine (See Bromocriptine).
Serotonin syndrome. Anecdotal case reports suggest benefit with cyproheptadine (Periactin), 12 mg orally (PO) initially, followed by 4 mg every hour for 3–4 doses (See Cyproheptadine). Chlorpromazine, 25–50 mg IV, has also been used.
Seizures
Assessment. Seizures are a major cause of morbidity and mortality from drug overdose or poisoning. Seizures may be single and brief or multiple and sustained and may result from a variety of mechanisms (Table I–13).
Table I-13 Selected Drugs and Toxins Causing Seizuresa
Table I-13 Selected Drugs and Toxins Causing Seizuresa
Adrenergic-sympathomimetic agents
Amphetamines and derivatives (including MDMA)
Caffeine
Cocaine
Ephedrine
Phencyclidine (PCP)
Phenylpropanolamine
Theophylline
Others
Antihistamines (diphenhydramine, hydroxyzine)
Beta receptor antagonists (primarily propranolol; not reported for atenolol, metoprolol, pindolol, or practolol)
Boric acid
Camphor
Carbamazepine
Cellular hypoxia (eg, carbon monoxide, cyanide, hydrogen sulfide)
Chlorinated hydrocarbons
Cholinergic agents (carbamates, nicotine, organophosphates)
Cicutoxin (water hemlock) and other plant toxins
Citrate
DEET (diethyltoluamide) (rare)
Ethylene glycol
Fipronil
Fluoride
Foscarnet
GHB (gamma hydroxybutyrate)
Isoniazid (INH)
Lamotrigine
Lead and other heavy metals
Lidocaine and other local anesthetics
Lithium
Mefenamic acid
Meperidine (normeperidine metabolite)
Metaldehyde
Methanol
Methyl bromide
Phenols
Phenylbutazone
Piroxicam
Salicylates
Strychnine (opisthotonus and rigidity)
Tiagabine
Tramadol
Withdrawal from ethanol or sedative-hypnotic drugs
Antidepressants and antipsychotics
Amoxapine
Bupropion
Haloperidol and butyrophenones
Loxapine, clozapine, and olanzapine
Phenothiazines
Tricyclic antidepressants
Venlafaxine other serotonin reuptake inhibitors
Generalized seizures usually result in loss of consciousness, often accompanied by tongue biting and fecal and urinary incontinence.
Other causes of muscular hyperactivity or rigidity (See Other Complications) may be mistaken for seizures, especially if the patient is also unconscious.
Complications
Any seizure can cause airway compromise, resulting in apnea or pulmonary aspiration.
Multiple or prolonged seizures may cause severe metabolic acidosis, hyperthermia, rhabdomyolysis, and brain damage.
Differential diagnosis. Rule out the following:
Any serious metabolic disturbance (eg, hypoglycemia, hyponatremia, hypocalcemia, or hypoxia).
Head trauma with intracranial injury.
Idiopathic epilepsy.
Withdrawal from alcohol or a sedative-hypnotic drug.
Exertional or environmental hyperthermia.
CNS infection such as meningitis or encephalitis.
Febrile seizures in children.
Treatment
Maintain an open airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Administer naloxone (See Naloxone and Nalmefene) if seizures are thought to be caused by hypoxia resulting from opioid-associated respiratory depression.
Check for hypoglycemia and administer dextrose and thiamine as for coma (See Altered Mental Status).
Use one or more of the following anticonvulsants. Caution: Anticonvulsants can cause hypotension, cardiac arrest, or respiratory arrest if administered too rapidly.
Diazepam, 0.1–0.2 mg/kg IV (See Benzodiazepines (Diazepam, Lorazepam, and Midazolam)).
Lorazepam, 0.05–0.1 mg/kg IV (See Benzodiazepines (Diazepam, Lorazepam, and Midazolam)).
Midazolam, 0.1–0.2 mg/kg IM (useful when IV access is difficult) or 0.05–0.1 mg/kg IV (See Benzodiazepines (Diazepam, Lorazepam, and Midazolam)).
Phenobarbital, 10–15 mg/kg IV; slow infusion over 15–20 minutes (See Phenobarbital).
Pentobarbital, 5–6 mg/kg IV; slow infusion over 8–10 minutes, then continuous infusion at 0.5–3 mg/kg/h titrated to effect (See Pentobarbital).
Propofol, 2–2.5 mg/kg IV (children: 2.5–3.5 mg/kg), infused in increments (40 mg at a time in adults) IV every 10–20 seconds until desired effect (See Propofol).
Phenytoin, 15–20 mg/kg IV; slow infusion over 25–30 minutes (See Phenytoin and Fosphenyton). Note: Phenytoin is ineffective for convulsions caused by theophylline and is considered the anticonvulsant of last choice for most drug-induced seizures.
Immediately check the rectal or tympanic temperature and cool the patient rapidly (See Seizures) if the temperature is above 40°C (104°F). The most rapid and reliably effective method of temperature control is neuromuscular paralysis with vecuronium, 0.1 mg/kg IV (See Neuromuscular Blockers) or another nondepolarizing neuromuscular blocker. Caution: If paralysis is used, the patient must be intubated and ventilated; in addition, monitor the electroencephalogram for continued brain seizure activity because peripheral muscular convulsions are no longer visible.
Use the following specific antidotes if available:
Pyridoxine (See Pyridoxine (Vitamin B6)) for isoniazid (INH; See Isoniazid (INH)).
Pralidoxime (2-PAM; See Pralidoxime and Other Oximes) or atropine (See Atropine and Glycopyrrolate) or both for organophosphate or carbamate insecticides (See Organophosphorus and Carbamate Insecticides).
Agitation, delirium, or psychosis
Assessment. Agitation, delirium, or psychosis may be caused by a variety of drugs and toxins (Table I–14). In addition, such symptoms may result from a functional thought disorder or metabolic encephalopathy caused by medical illness.
Table I-14 Selected Drugs and Toxins Causing Agitation, Delirium, or Confusiona
Table I-14 Selected Drugs and Toxins Causing Agitation, Delirium, or Confusiona
Predominant confusion or delirium
Predominant agitation or psychosis
Amantadine
Amphetamines and derivatives
Anticholinergic agents
Caffeine
Antihistamines
Cocaine
Bromide
Cycloserine
Carbon monoxide
Dextromethorphan
Cimetidine
LSD (lysergic acid diethylamide)
Disulfiram
Marijuana
Lead and other heavy metals
Mercury
Levodopa
Phencyclidine (PCP)
Lidocaine and other local anesthetics
Procaine
Lithium
Serotonin reuptake inhibitors (SSRIs)
Salicylates
Steroids (eg, prednisone)
Withdrawal from ethanol or sedative-hypnotic drugs
Theophylline
Functional psychosis or stimulant-induced agitation and psychosis are usually associated with an intact sensorium, and hallucinations are predominantly auditory.
With metabolic encephalopathy or drug-induced delirium, there is usually alteration of the sensorium (manifested by confusion or disorientation). Hallucinations, when they occur, are predominantly visual. Anticholinergic delirium is often accompanied by tachycardia, dilated pupils, flushing, dry skin and mucous membranes, decreased peristalsis, and urinary retention.
Complications. Agitation, especially if accompanied by hyperkinetic behavior and struggling, may result in hyperthermia (See Hyperthermia) and rhabdomyolysis (See Rhabdomyolysis).
Differential diagnosis. Rule out the following:
Serious metabolic disturbance (hypoxia, hypoglycemia, or hyponatremia).
Alcohol or sedative-hypnotic drug withdrawal.
Thyrotoxicosis.
CNS infection such as meningitis or encephalitis.
Exertion-induced or environmental hyperthermia.
Treatment. Sometimes, the patient can be calmed with reassuring words and reduction of noise, light, and physical stimulation. If this is not quickly effective, rapidly gain control of the patient to determine the rectal or tympanic temperature and begin rapid cooling and other treatment if needed.
Maintain an open airway and assist ventilation if necessary (See Airway). Administer supplemental oxygen.
Treat hypoglycemia (See Hypernatremia and hyponatremia), hypoxia (See Hypoxia), or other metabolic disturbances.
Administer one of the following benzodiazepines:
Midazolam, 0.05–0.1 mg/kg IV over 1 minute, or 0.1–0.2 mg/kg IM (See Benzodiazepines (Diazepam, Lorazepam, and Midazolam)).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree