FIGURE 1 Two sources represented as dipoles with different orientations relative to the scalp surface. An electrical current generates a dipole; two opposite polarities. Tangential dipoles are parallel to scalp surface and radial dipoles are perpendicular to scalp surface. MEG is sensitive to tangential dipoles, while EEG is sensitive to both tangential and radial dipoles. Thus, EEG picks up more neural activity that emerges from the brain than MEG.
Practically, for recording EEG and MEG two approaches can be used: 1) recording of the spontaneous EEG/MEG activity and 2) recording EEG/MEG responses to sensory stimulation.
The spontaneous EEG/MEG is typically measured when subjects are awake but not engaged in a task. A common way to analyze the spontaneous EEG/MEG is to transform the EEG/MEG signal into the frequency domain where the relative dominance of each frequency in the EEG/MEG signal is computed. This frequency versus amplitude illustration is also termed power spectrum [5]. Differences in amplitude within different frequency bands are thought to reflect functionally different states of the cortex. For example, a reduction of amplitude in the alpha band (8–12 Hz) is thought to reflect a state of cortical activation [19].
The EEG responses to an event or stimulus are called Event-Related Potentials (ERPs). The MEG responses are called Event-Related magnetic Fields (ERFs). ERPs are the voltage changes in the EEG that are time-locked to the onset of the stimulus [3]. Likewise, ERFs are time-locked changes in the magnetic field. Practically, ERPs/ERFs are obtained by delivering the same stimulus a number of times. Then, the EEG/MEG is segmented into epochs relative to stimulus onset and averaged across all trials. The reason for giving multiple stimuli is that the magnitude of the ERP/ERF amplitude is low in comparison with the background EEG/MEG. Across-trial averaging enhances the signal-to-noise ratio of signal changes that are time-locked to the onset of the stimulus (i.e. ERPs/ERFs relative to ongoing EEG/MEG activity and background noise) [17].
HUMAN SURROGATE MODEL OF ACUTE POSTOPERATIVE PAIN
Acute postoperative pain is typically accompanied by increased pain sensitivity (i.e. hyperalgesia) in and surrounding the region subjected to surgical damage. One aspect contributing to this postsurgical pain and hyperalgesia is the tissue damage due to the surgical incision. In order to achieve a better understanding of the development of this incision-induced pain and hyperalgesia, an experimental incision model has been developed [4,7,8]. In this model, a 4 mm length incision is made through the skin, fascia, and muscle. When performed, this incision induces a moderate to strong ongoing pain which then progressively declines. Directly after the incision, the skin develops a flare reaction in the skin surrounding the injured area, suggesting inflammation and the activation of peptidergic nociceptive afferents. Two types of cutaneous hyperalgesia are observed: (1) hyperalgesia at the site of incision referred to as “primary” hyperalgesia and characterized by reduced thresholds to heat and mechanical punctate stimuli, and (2) hyperalgesia in the skin surrounding the incision, referred to as “secondary” hyperalgesia and characterized by reduced thresholds and increased perceived intensity to mechanical punctate stimuli [4]. This secondary hyperalgesia is considered to be a key feature of what is called “central sensitization” [31]. This because it has been shown that once it has developed it appears to be less dependent or even independent of peripheral neural activity [7,8].
However, making an experimental skin incision in healthy volunteers is invasive. Therefore, an alternative method which avoids skin tissue damage, is high frequency electrical stimulation of the skin [9]. HFS consists of five trains of 100 Hz delivered at 10 or 20 x the detection threshold during 1 sec at a 10 sec inter-train interval and results in: (1) a flare formation in the skin surrounding the stimulated area, (2) enhanced pain perception to electrical nociceptive test stimuli applied to the conditioned area (“homotopic” hyperalgesia) and (3) reduced thresholds and increased perceived intensity to mechanical punctate stimuli applied to the skin surrounding the conditioned area (“heterotopic” hyperalgesia) [9,10,26,27,30]. Note that this heterotopic hyperalgesia shares many similarities with the “secondary” hyperalgesia observed after experimental incision (Fig. 2).
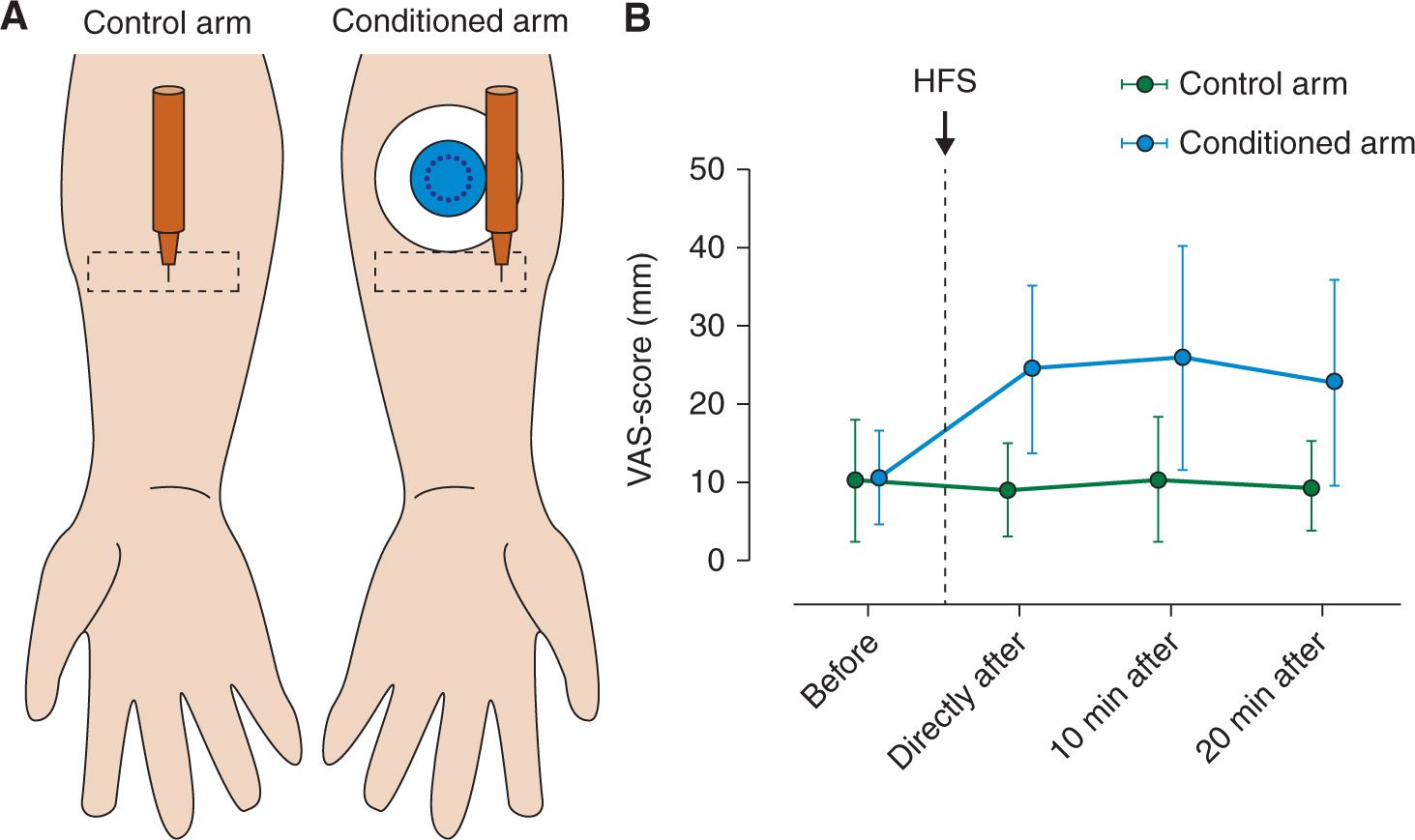
FIGURE 2 Heterotopic mechanical punctate hyperalgesia induced by HFS. (A) A calibrated punctate probe (256 mN) is used to stimulate the heterotopic skin (rectangular shape) before and after HFS, delivered by the conditioning electrode (circular object with black dots in the middle) (B) Pain intensity ratings in response to the pinprick stimulation increased on the conditioned arm after HFS (Modified from Van Den Broeke EN, Geene N, Van Rijn CM, et al. Negative expectations increases mechanical hyperalgesia after high frequency conditioning stimulation of human skin. Eur J Pain 2014; 18 (1): 86–91).
ERPS AND ERFS: CORRELATES OF EXPERIMENTALLY INDUCED SECONDARY HYPERALGESIA IN HEALTHY VOLUNTEERS AND PATIENTS
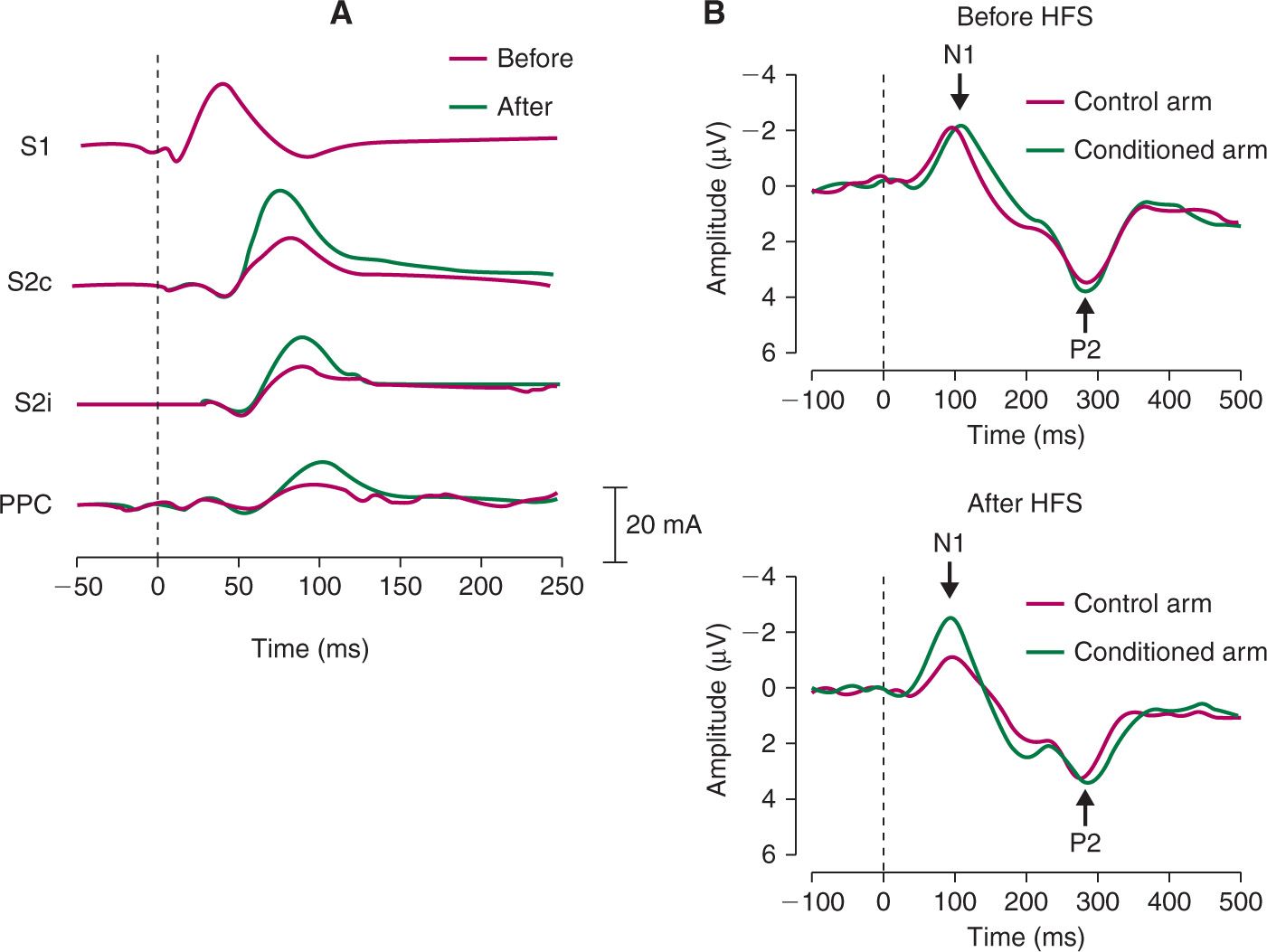
In order to investigate brain correlates of secondary mechanical hyperalgesia, Maihöfner et al. [13] recorded MEG in response to mechanical punctate stimuli before and after painful electrical conditioning stimulation. The mechanical punctate stimuli were delivered to the area surrounding the conditioned area via a pneumatically driven von Frey probe (256 mN). After conditioning stimulation, mechanical punctate stimuli applied in the area of secondary hyperalgesia elicited enhanced brain responses, i.e., enhanced event-related magnetic fields between 70 and 110 ms after stimulus onset (Fig. 3A). The authors further showed that, within this latency window, multiple cortical sources showed enhanced brain responses including the secondary somatosensory cortex (contralateral and ipsilateral) and the posterior parietal cortex. Recently, Iannetti et al. [6] recorded EEG responses to mechanical pinprick stimulation applied in the area of secondary hyperalgesia induced by intradermal capsaicin injection. Capsaicin injection induces a strong painful burning sensation for several seconds and then progressively attenuates. After capsaicin injection, mechanical punctate stimuli elicited enhanced brain responses i.e., the magnitude of the elicited event-related potentials, was increased between 70 and 110 ms. Interestingly, similar results were observed after HFS with pinprick-like electrical stimuli delivered using a concentric electrode [27]. Indeed, electrical stimuli, applied to the skin adjacent to the conditioned area elicited enhanced event-related potentials between 70 and 110 ms (Fig. 3B). Surprisingly, we did not observe a similar pattern in the behavioral responses, that is, the intensity of the percept elicited by the electrical stimuli was not affected by HFS. A possible explanation to this discrepancy might be that secondary hyperalgesia is specific for mechanical nociceptive input [10,14,32]. An electrical stimulus activates multiple types of afferent fibers and its percept probably results from the integration of afferent inputs conveyed through different categories of fibers. On the other hand, although it has been shown that a mechanical punctate stimulus is able to activate nociceptors [24] it inevitably also activates low threshold mechanoreceptors (LTMs). Nevertheless, the results of Van den Broeke et al. [27] show that the EEG correlate of secondary hyperalgesia can be measured in absence of its behavioural correlate, and that measuring brain activity can provide additional information which is not obtained by measuring perception alone.