A basic knowledge of cerebral and spinal cord physiology and the effects of anesthetic drugs on this physiology is necessary to make informed anesthetic choices for neurosurgical procedures.
 Barbiturates cause a dose-dependent reduction in cerebral blood flow (CBF) and cerebral metabolic rate (CMRO2), with the CMRO2 reduction due to the metabolic component associated with electrical brain function (as opposed to that associated with maintenance of cellular integrity). Large doses are capable of producing electroencephalogram (EEG) burst suppression, and they may be used clinically for this purpose. The intracranial pressure (ICP) reduction associated with thiopental has historically made this drug an integral part of neuroanesthesia practice.
Barbiturates cause a dose-dependent reduction in cerebral blood flow (CBF) and cerebral metabolic rate (CMRO2), with the CMRO2 reduction due to the metabolic component associated with electrical brain function (as opposed to that associated with maintenance of cellular integrity). Large doses are capable of producing electroencephalogram (EEG) burst suppression, and they may be used clinically for this purpose. The intracranial pressure (ICP) reduction associated with thiopental has historically made this drug an integral part of neuroanesthesia practice.
 Propofol has become the popular alternative to thiopental for induction in the neurosurgical patient. Like barbiturates, it is associated with a reduction in CBF, CMRO2, and ICP. The reduction in mean arterial pressure (MAP) associated with bolus doses of propofol necessitates caution in the elderly and volume-depleted patient. The favorable pharmacokinetic profile of propofol allows for prompt awakening following a continuous infusion, which makes this drug useful for intubated patients in whom sedation is desirable but intermittent evaluation of neurologic status is also required.
Propofol has become the popular alternative to thiopental for induction in the neurosurgical patient. Like barbiturates, it is associated with a reduction in CBF, CMRO2, and ICP. The reduction in mean arterial pressure (MAP) associated with bolus doses of propofol necessitates caution in the elderly and volume-depleted patient. The favorable pharmacokinetic profile of propofol allows for prompt awakening following a continuous infusion, which makes this drug useful for intubated patients in whom sedation is desirable but intermittent evaluation of neurologic status is also required.
 Narcotics lack adverse effects on CBF, CMRO2, and ICP as long as ventilation is maintained and thus have remained a valuable part of the neuroanesthetic regimen. Fentanyl has been the narcotic of choice for most neuroanesthetics, but remifentanil has been added as a popular choice for many procedures. The context-sensitive half-time of remifentanil makes infusion of this drug an excellent choice to provide profound analgesia while maintaining the ability to awaken the patient promptly to evaluate neurologic status.
Narcotics lack adverse effects on CBF, CMRO2, and ICP as long as ventilation is maintained and thus have remained a valuable part of the neuroanesthetic regimen. Fentanyl has been the narcotic of choice for most neuroanesthetics, but remifentanil has been added as a popular choice for many procedures. The context-sensitive half-time of remifentanil makes infusion of this drug an excellent choice to provide profound analgesia while maintaining the ability to awaken the patient promptly to evaluate neurologic status.
 Desflurane and sevoflurane have cerebral effects very similar to those of isoflurane, with the potential to cause cerebrovasodilation and an increase in CBF and ICP, particularly when used in higher doses. The reduction in CMRO2 associated with these drugs is dose related, with a maximal reduction attained with the occurrence of EEG suppression. Despite the potential to increase CBF and ICP, these drugs are commonly used in neuroanesthesia in combination with other drugs that have more desirable cerebral effects. However, in patients in whom intracranial compliance is exhausted, the dose of these drugs is kept low (~1 minimum alveolar concentration [MAC] or less), or they are eliminated completely from the anesthetic regimen.
Desflurane and sevoflurane have cerebral effects very similar to those of isoflurane, with the potential to cause cerebrovasodilation and an increase in CBF and ICP, particularly when used in higher doses. The reduction in CMRO2 associated with these drugs is dose related, with a maximal reduction attained with the occurrence of EEG suppression. Despite the potential to increase CBF and ICP, these drugs are commonly used in neuroanesthesia in combination with other drugs that have more desirable cerebral effects. However, in patients in whom intracranial compliance is exhausted, the dose of these drugs is kept low (~1 minimum alveolar concentration [MAC] or less), or they are eliminated completely from the anesthetic regimen.
 Nitrous oxide has the potential to increase CBF and ICP with no change or an increase in CMRO2. The recent use of desflurane and sevoflurane in neuroanesthesia, which have low blood-gas solubility coefficients and allow for more rapid awakening, has lessened the usefulness of nitrous oxide in neuroanesthesia.
Nitrous oxide has the potential to increase CBF and ICP with no change or an increase in CMRO2. The recent use of desflurane and sevoflurane in neuroanesthesia, which have low blood-gas solubility coefficients and allow for more rapid awakening, has lessened the usefulness of nitrous oxide in neuroanesthesia.
 Muscle relaxants do not cross the blood–brain barrier and thus central nervous system (CNS) effects are secondary to systemic effects (such as histamine release). Vecuronium, rocuronium, and cis-atracurium are most commonly chosen, and these do not affect CBF or ICP. Succinylcholine causes a mild, transient increase in ICP. The ability of succinylcholine to cause significant hyperkalemia in certain neurosurgical patients (plegic patients, those with neuromuscular diseases, etc.) limits its use more than its effects on CBF or ICP.
Muscle relaxants do not cross the blood–brain barrier and thus central nervous system (CNS) effects are secondary to systemic effects (such as histamine release). Vecuronium, rocuronium, and cis-atracurium are most commonly chosen, and these do not affect CBF or ICP. Succinylcholine causes a mild, transient increase in ICP. The ability of succinylcholine to cause significant hyperkalemia in certain neurosurgical patients (plegic patients, those with neuromuscular diseases, etc.) limits its use more than its effects on CBF or ICP.
The anesthetic management of neurosurgical patients is based on the knowledge of the selected drug’s influence on the CNS physiology. The specific anesthetic regimen is a combination of drugs that favorably affects cerebral hemodynamics, cerebral metabolism, and ICP to provide good operating conditions and to enhance the probability of a quality outcome. Most anesthetic drugs have been studied in this regard and, as new drugs are developed, their effects on cerebral physiology will be elucidated. The effects of anesthetic drugs on cerebrospinal fluid (CSF) volume (as determined by the rate of formation and the resistance to reabsorption) have also been determined.
Fewer studies have addressed the effects of anesthetic drugs on spinal cord physiology. This could be in part because non-invasive methods of measuring various aspects of spinal cord physiology in the human are not available so that most of the data have been derived from animal studies. Although it has been assumed that the effects of anesthetics on the spinal cord mimic their effects in the brain (and this is likely to be qualitatively correct), to make true comparisons, investigators must examine both brain and spinal cord parameters simultaneously.
I. INTRAVENOUS DRUGS
A. Barbiturates
1. Effect on CBF and cerebral oxygen consumption (Table 2.1). Barbiturates were the first anesthetics to be examined for their cerebral vascular effects. Thiopental decreases CBF and cerebral metabolic rate for oxygen consumption (CMRO2) in a parallel fashion up to the point of isoelectricity on the EEG. The changes in CBF are thought to be secondary to the changes in CMRO2 (a coupled decrease in flow and metabolism). The component of CMRO2 that is affected is related to electrical brain function; there is minimal effect on the component of CMRO2 associated with cellular homeostasis. At the point at which an isoelectric EEG occurs after the administration of thiopental, an approximately 50% decrease in CMRO2 occurs with no cerebral metabolic evidence of toxicity. If barbiturates are used clinically for the purpose of cerebral protection, the endpoint of EEG burst suppression is often used to provide near-maximal metabolic suppression. The reduction in MAP associated with the high doses of thiopental needed to provide EEG burst suppression may require concomitant use of a vasopressor to maintain cerebral perfusion pressure (CPP), the difference between MAP and ICP. Methohexital differs from other barbiturates in regard to epileptiform activity in that it may induce seizures in patients who have epilepsy. If seizure activity occurs with methohexital, this results in an increase in CMRO2 and CBF.
TABLE 2.1 Effective of Anesthetic Drugs on Cerebral Blood Flow, Cerebral Metabolic Rate for Oxygen Consumption, and Intracranial Pressure
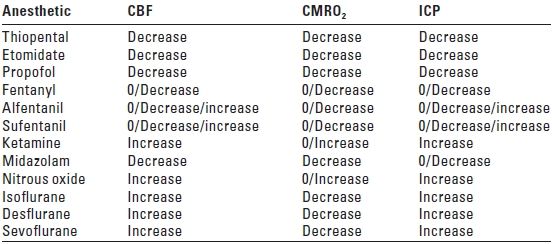
CBF, cerebral blood flow; CMRO2; cerebral metabolic rate for oxygen consumption; ICP, intracranial pressure.
2. Effect on autoregulation and CO2 reactivity. Thiopental, even in high doses, does not appear to abolish cerebral autoregulation or CO2 reactivity.
3. Effect on CSF dynamics (Table 2.2). Low doses of thiopental cause no change in the rate of CSF formation (Vf) and either no change or an increase in the resistance to reabsorption of CSF (Ra). This would predict no change or an increase in ICP. High doses of thiopental cause a decrease in Vf and either no change or a decrease in Ra with a predicted decrease in ICP.
TABLE 2.2 Effects of Intravenous Drugs on Rate of Cerebrospinal Fluid Formation, Resistance to Reabsorption of Cerebrospinal Fluid, and the Predicted Effect on Intracranial Pressure
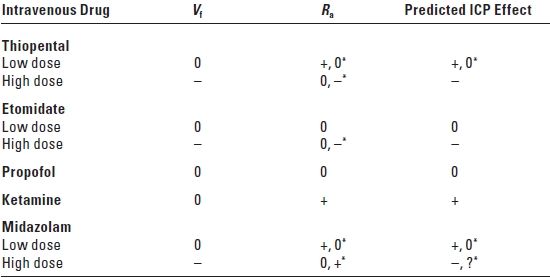
Vf, rate of CSF formation; Ra, resistance to CSF reabsorption; ICP, intracranial pressure; 0, no change; +, increase; –, decrease; *, effect dependent on dose; ?, uncertain.
Adapted from Artru AA, CSF dynamics, cerebral edema, and intracranial pressure. In: Albin MS, ed. Textbook of Neuroanesthesia: With Neurosurgical and Neuroscience Perspectives. New York, NY: McGraw-Hill, 1997:61.
4. Effect on ICP. As a result of the reduction in both CBF and cerebral blood volume (CBV), barbiturates lower ICP. Barbiturates are used clinically for this purpose and may even be effective when other methods for reducing ICP have failed.
5. Effect on spinal cord blood flow (SCBF) and metabolism. Barbiturates produce a significant reduction in SCBF. Autoregulation of SCBF remains intact under barbiturate anesthesia (as demonstrated with thiopental) with an autoregulatory range of approximately 60 to 120 mm Hg. Pentobarbital has been shown to decrease local utilization of glucose in the spinal cord, although the magnitude of this effect is smaller than that seen in the brain.
B. Etomidate
1. Effect on CBF and CMRO2. Etomidate, like the barbiturates, reduces CBF and CMRO2. An isoelectric EEG can be induced with etomidate, and, as with thiopental, there is no evidence of cerebral toxicity as reflected by normal brain metabolites. In addition, no further reduction in CMRO2 occurs when additional doses are given after EEG burst suppression is achieved. Myoclonus produced by the drug has the disadvantage of being misinterpreted as seizure activity in neurosurgical patients. The use of etomidate may suppress the adrenocortical response to stress; however, this may not be an issue in patients who have intracranial tumors because they usually are already receiving steroids. Less cardiovascular depression with etomidate as compared to thiopental or propofol makes this drug advantageous for the induction of anesthesia in trauma patients who have a low blood volume and older neurosurgical patients who have multiple medical problems.
2. Effect on autoregulation and CO2 response. Reactivity to CO2 is maintained with the administration of etomidate. The effect of etomidate on autoregulation has not been evaluated.
3. Effect on CSF dynamics. Low-dose etomidate causes no change in Vf and Ra with no predicted effect on ICP. High-dose etomidate causes a decrease in Vf and either no change or a decrease in Ra with a predicted decrease in ICP.
4. Effect on ICP. Etomidate has been shown to reduce ICP without decreasing CPP and is clinically useful in neurosurgical patients for this purpose.
C. Propofol
1. Effect on CBF and CMRO2. Propofol produces dose-related reductions in both CBF and CMRO2. The pattern of regional CBF (rCBF) changes is different for propofol and thiopental. Using positron emission tomography (PET) in adult volunteers, propofol has been shown to decrease rCBF in the anterior brain regions, whereas thiopental decreases rCBF primarily in the cerebellar and posterior brain regions.
A disadvantage of large bolus doses of propofol is a reduction in MAP, which can decrease CPP. In neurosurgical patients who are hypovolemic, the reduction in MAP might be substantial and either the intravascular volume of these patients should be restored before the administration of propofol or an alternative induction drug should be used. The more frequent maintenance of normovolemia in recent years in neurosurgical patients has made this less of an issue. A continuous infusion of propofol may be used intraoperatively as part of a total intravenous technique. The combination of an infusion of propofol and a narcotic (usually remifentanil) is particularly useful when the monitoring of evoked potentials precludes the use of other than low concentrations of inhalational drugs. The technique using propofol and remifentanil infusions along with a low-dose inhalational drug is also useful for providing good operating conditions when ICP is elevated. Another frequent use of propofol during neurosurgery is for sedation during awake craniotomies.
2. Effect on autoregulation and CO2 response. Autoregulation and CO2 response are preserved during the administration of propofol.
3. Effect on CSF dynamics. Propofol causes no change in Vf or Ra with no predicted effect on ICP.
4. Effect on ICP. Propofol reduces ICP. Because it also reduces MAP, its effect on CPP must be carefully monitored. Nonetheless, propofol’s ICP-lowering effect makes it useful in the intensive care unit (ICU) for the sedation of patients in whom elevated ICP is a concern. Propofol has the advantage of allowing prompt awakening, which is advantageous in patients whose neurologic status needs to be evaluated serially. In the operating room, moderately deep sedation with propofol does not increase ICP in comparison to no sedation in patients undergoing stereotactic biopsy for brain tumors. During craniotomy for resection of brain tumors, ICP has been shown to be lower in patients who receive propofol–fentanyl in comparison to patients anesthetized with isoflurane–fentanyl or sevoflurane–fentanyl. The antinausea effect of propofol is also advantageous in neurosurgical patients because many of them receive moderate to large doses of narcotics, which are associated with a high incidence of nausea and vomiting. This can be particularly deleterious because nausea-induced retching and vomiting might increase ICP. Careful attention to sterile technique is essential when using propofol as an infusion because the solubilizing drug in which propofol is prepared provides an excellent medium for bacterial growth.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






