Control
Loss of control
Large amount of food eaten
Overeating
Objective binge eating
Average amount of food eaten
Normal eating
Subjective binge eating
10.5.3 Bulimia Nervosa
Although seen less frequently in obese individuals than BED and NES, BN does occur in obese individuals [55] and thus should be assessed. In addition to regular binge eating episodes as defined in BED, a diagnosis of BN requires the use of problematic methods to prevent weight gain such as inducing vomiting, misuse of laxatives, prolonged fasting, and excessive exercise. The frequency of binge episodes and compensatory behaviors occur at least weekly and symptoms persist for at least 3 months .
10.5.4 Night Eating Syndrome
Whereas the core feature of BED is episodes of overeating accompanied by a lack of control without the use of inappropriate compensatory behaviors, the core feature of NES is the delayed circadian shift of eating [8, 56]. First described by Stunkard et al. in 1955 [57], awareness of NES amongst clinicians and researchers has only recently emerged. It is now listed in the DSM-5 as an Other Specified Feeding or Eating Disorder but diagnostic criteria have not yet been fully quantified. Allison et al. [28] propose that for NES, as established by the International NES Working group, the core criterion is an abnormally increased food intake in the evening and/or at night, manifested by (1) consumption of at least 25 % of intake after the evening meal and before bedtime and/or (2) nocturnal awakenings with eating at least twice per week. In addition to distress or impairment in functioning, NES involves awareness of the eating episodes as this feature differentiates NES from sleep-related eating disorder (SRED) , a parasomnia in which individuals do not recall their night-time eating [28]. Allison et al. [28] propose additional criteria for NES that involves the endorsement of at least three of five additional symptoms: (1) lack of eating in the morning; (2) strong desire to eat between dinner and sleep or upon awakening from sleep; (3) insomnia; (4) belief that one must eat in order to get to sleep; and (5) low mood in the evening. These criteria must be met for a minimum duration of 3 months. These additional criteria are not required by the DSM-5 given that further research on night eating is needed; however, the criteria outlined by Allison et al. [28] are useful for differentiating NES from other disorders such as BED and SRED .
10.5.5 Differentiating Between BED and NES
BED and NES are thought to be distinct disorders; however, they share the common feature of evening hyperphagia ([28], see Fig. 10.1). The rates of co-occurring BED and NES range from 7 to 25 % and approximately 9 % for BN and NES [28, 58].
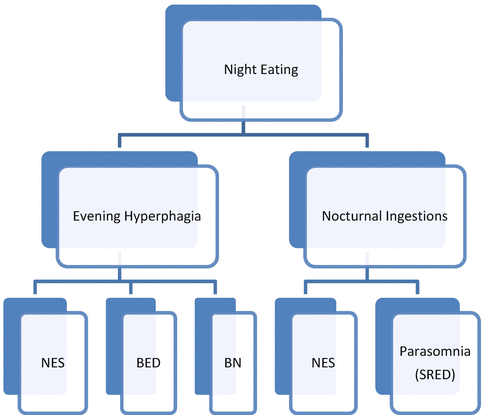

Fig. 10.1
Differentiating NES from other eating and sleep disorders
NES can be distinguished from BED in that individuals with NES demonstrate a phase delay in the circadian pattern of eating leading them to consume more of their total energy intake later in the day and at night compared to individuals without NES [36, 46, 59]. Gluck et al. [46] found that after fasting for 8 h, night eaters reported less hunger before a daytime test meal. In terms of waking to eat, Birketvedt et al. [59] found that night eaters woke on average 3.6 times per night compared to only 0.3 times in controls and 52 % of awakenings in night eaters were accompanied by food intake. NES participants have been found to have lower disinhibition scores than individuals with BED, but higher than controls [60], and those with both BED and NES scored the highest. One difference that has been highlighted in the literature is that sleep disturbance is less common in individuals with BED compared to those with NES [4, 36].
Individuals with NES have also been found to exhibit less general psychological distress than individuals with BED [8]. Allison et al. [37] in their study comparing individuals with BED and NES found the BED group exhibited greater eating pathology and experienced many more objective binge eating episodes with loss of control. Some of the NES participants reported a loss of control during their night eating episodes, but this was rarely objectively large in terms of quantity of food. The eating patterns of the groups differed significantly as well, with the NES group eating far fewer meals during the first half of the day. The BED and NES groups ate a similar number of evening snacks after the main evening meal, indicating that both groups have some trouble regulating their eating in the evening hours before bedtime. The BED group had higher scores on the Eating Disorders Exam-Questionnaire ([61], please see Table 10.3 for a description of this measure) weight concern and shape concerns subscales than the NES group even after controlling for BMI differences. Allison et al. [37] note that since both the NES and BED groups were characterized by disordered eating, clinicians should attend to the assessment and regulation of meal patterns. For nocturnal ingestions, NES can also be distinguished from sleep-related eating disorder in that individuals with NES are awake when eating and can recall these episodes compared to individuals with SRED who are asleep when these eating episodes occur [5].
10.5.6 Avoidant Restrictive Food Intake Disorder
ARFID is characterized by an apparent lack of interest in eating or food, avoidance of food based on the food’s sensory characteristics, and avoidance of particular foods due to concerns about the negative consequences of eating, like abdominal discomfort. Unlike other eating disorders, the motivation to avoid eating is not to achieve or maintain a certain body weight or shape. ARFID can co-occur with a number of other mental disorders, in particular, with autism spectrum disorder and other neurodevelopmental disorders, as well as anxiety disorders [62]. Little research on ARFID in adults has been published and the prevalence of ARFID in obese individuals has not been documented. However, sufficient numbers of post-bariatric surgery patients across different countries and settings have been documented with problematic food aversions to have generated several publications and research studies (e.g., [63–67]. Although these studies do not diagnose these individuals with ARFID, the presentation may be similar to that of ARFID. Foods that are typically avoided after bariatric surgery tend to be tougher or denser in texture, particularly meats, rice, and pasta. These aversions usually develop after the foods in question lead to an episode of abdominal discomfort. However, these food avoidances tend to disappear over time and do not usually indicate a diagnosis of ARFID .
10.5.7 Assessment Methods
As with other psychiatric disorders, the eating disorders involve symptoms that are behavioral (e.g., bingeing, restriction), cognitive (e.g., prioritization of dietary restraint, negative body image), and affective (e.g., eating to regulate emotional distress) in nature and a complete, valid, and reliable assessment must consider all of these. Table 10.2 lists the symptoms and features that are typically found in eating disorders that should be assessed in all patients.
Table 10.2
Characteristic features and symptoms by DSM-5 disorder , to be assessed in all patients
Feature | BED | BN | NES | ARFID |
|---|---|---|---|---|
Objective binge eating | ✓ | ✓ | ||
Loss of control over eating | ✓ | ✓ | ✓ | |
Extreme compensatory methods | ✓ | |||
Overvaluation of weight and shape | ✓ | ✓ | ||
Excessive evening eating | ✓ | ✓ | ✓ | |
Nocturnal eating upon awakening | ✓ | |||
Irregular/chaotic eating pattern | ✓ | ✓ | ✓ | ✓ |
Dietary restriction | ✓ | ✓ | ✓ | ✓ |
Regardless of whether the assessment is conducted for treatment or research purposes, a clinical interview should be the foundation of any valid eating disorders assessment. Based on the available research evidence and our clinical experience, clinician interviews are critical for understanding past and present eating behaviors and for obtaining accurate diagnoses in both bariatric surgery [68–71] and non-bariatric surgery populations [61]. The choice of particular interview and questionnaire instruments can then be based on factors such as time available to conduct the assessment and the type of information that is useful in a particular setting. Please see Table 10.3 for a description of the recommended eating disorder-specific interview and self-report assessment instruments.
Table 10.3
Recommended instruments
Measure | Description |
|---|---|
Clinical interview | |
Eating disorder examination—interview [54] | Takes ~45–75 min and is freely available; diagnoses eating disorders according to the DSM-IV and provides scores on eating disorder pathology via four subscales: dietary restraint, eating concern, weight concern, shape concern, plus a global score are computed |
Night eating syndrome history and inventory (NESHI) [78] | A semi-structured interview used to diagnose NES. It assesses a typical 24-h food intake, including a recall of all meals and snacks, and sleeping patterns. A total score of 25 can be used as the threshold for diagnosing NES |
Self-report | |
Binge eating scale [124] | 16 items; computes a total score reflecting severity of binge behaviors to screen for binge eating disorder; measures the behavioral, emotional, and cognitive symptoms associated with binge eating. Participants with scores ≥17 can be classified “binge eaters.” [131]. Cut scores of binge eating severity [132]: no binge eating (score ≤17), mild to moderate binge eating (score of 18–26), severe binge eating (score ≥27) |
The Dutch eating behaviors questionnaire [125] | 33 items, developed to measure aspects of eating found through research to be common in obese individuals: restrained eating, emotional eating, external eating, with emotional and external eating characteristic of individuals with obesity; not diagnostic |
Eating disorders exam—questionnaire [75] | 28 items; adapted from the EDE-I to assess symptoms of eating disorders according to the DSM-IV; four subscales: restraint, eating concern, shape concern, weight concern, and a global score |
Eating disorders inventory-3 [126] | 91 items organized into 12 primary scales: Drive for Thinness, Bulimia, Body Dissatisfaction, Low Self-Esteem, Personal Alienation, Interpersonal Insecurity, Interpersonal Alienation, Interoceptive Deficits, Emotional Dysregulation, Perfectionism, Asceticism, and Maturity Fears. Yields six composite scores: one that is eating disorder specific (i.e., Eating Disorder Risk) and five that are general integrative psychological constructs (i.e., Ineffectiveness, Interpersonal Problems, Affective Problems, Overcontrol, General Psychological Maladjustment) |
Questionnaire for eating disorder diagnoses [127] | 50 items that operationalize the DSM (fourth edition) eating disorder diagnoses into (a) those with and without an eating disorder diagnosis, (b) eating-disordered, symptomatic, and asymptomatic individuals, and (c) those with anorexia and bulimia diagnoses |
Questionnaire of eating and weight patterns (revised) [128] | 28 items; assesses symptoms and history of BED and BN as per the DSM-IV |
Night eating questionnaire [129] | 17 items; assesses presence and severity of symptoms of night eating |
Night eating diagnostic questionnaire [47] | 21 items to diagnose NES as per Allison et al. [30] |
Three-factor eating questionnaire/eating inventory [130] | 51 items; measures three aspects of eating behavior found in research to be common in individuals with obesity, through three scales: Cognitive restraint, disinhibition, hunger. High scores on disinhibition and hunger are characteristic of BED. Not diagnostic |
The Eating Disorders Exam-Interview [72] is the most used and widely recognized interview used to diagnose eating disorders. The eating disorders modules of the Structured Clinical Interview [73] and the MINI Neuropsychiatric Interview are also available [74]. However, as of yet these measures do not include questions to assess NES or ARFID. The Night Eating Syndrome History and Inventory (NESHI) is a more recent addition to the field and can be used to guide the assessment of NES [75]. Currently there are no validated instruments for assessing ARFID. Therefore, a thorough understanding of when and how the food aversion developed, the persistence of the avoidance and associated distress and/or impairment are needed to inform diagnosis.
Self-report instruments are useful to screen for particular disorders like binge eating disorder or night eating syndrome and features like shape- and weight-related concerns. However, binge eating, which is necessary for diagnosing BED and BN, is difficult to assess accurately through self-report instruments. The bulk of the research conducted on the concordance between self-report and interview measures have compared the Eating Disorders Exam-Interview (EDE-I) to the Eating Disorders Exam-Questionnaire (EDE-Q) [76–79]. In summary, this research shows that self-report measures yield higher levels of binge eating compared to clinical interviews [61, 76–80]. As Fairburn and Beglin [61] note and our experience supports, this is because patients generally lack the knowledge required for accurately defining a binge. The term “binge eating” has entered the cultural lexicon, but individuals who do not have an understanding of the clinically defined features of a binge, such as the amount of food required for an objective binge, cannot accurately report on these symptoms. Clinical interviews thus allow for the probing that is needed to determine whether a patient who reports “binge eating” truly is binge eating according to the DSM-5.
In addition to binge eating, self-report measures as compared to clinical interviews tend to yield higher scores on weight- and shape-related concerns [81]. Fairburn and Beglin [61] posit that similarly to binge eating, laypersons lack the knowledge needed to assess clinically defined weight- and shape-related distress. The ability to differentiate normative dissatisfaction about shape and weight from the more extreme concerns typical in eating disorders requires clinical experience. However, they found little discrepancy between the EDE-Q and EDE-I in assessing the frequency of self-induced vomiting and laxative misuse, likely because these are behaviors that are simply either present or absent. The overall pattern of findings in this study suggested that self-report questionnaires can be used to assess clearly defined behavioral symptoms like self-induced vomiting, laxative misuse, and dietary restraint, but interviews should be used to assess binge eating and body dissatisfaction .
10.6 Considerations for Triaging to Treatment
Once an obese patient has been diagnosed with an eating disorder, planning for treatment involves several considerations (see Table 10.4 for steps for triage following a diagnosis of an eating disorder).
Table 10.4
Steps for triage following diagnosis of an eating disorder
1. Complete diagnostic assessment |
2. Communicate diagnosis to patient |
3. Provide psychoeducation |
4. Assess motivation to change |
5. Consider medications |
6. Involve other health care providers |
7. Monitor medical status |
8. Refer to evidence-based psychotherapy |
10.6.1 Dieting
Weight loss diets are often a first choice option for obese individuals who want to lose weight; however, for individuals who are obese and have an eating disorder, there are some important cautions. Individuals who diet typically only lose 5–10 % of their total body weight [82–84]. Also, the long-term maintenance of weight loss is typically minimal with only approximately 7 lbs with diet alone or 8 lbs with diet and exercise [85–87]. There are numerous different types of diets available and there is no consensus on which diets work best; however, diet and exercise combined has been found to increase the likelihood of maintaining weight loss at 1 year [88]. Very low calorie diets demonstrate the greatest efficacy in terms of amount of initial weight loss (approximately 20 %), but these diets are difficult to sustain [83]. Long-term studies suggest that many dieters regain more weight than they lost initially and lifestyle changes alone have not been found to produce significant long-term weight reductions [82]. When examining individuals who are successful at maintaining weight loss, findings from the National Weight Control Registry have found that factors associated with weight loss maintenance include having a weight loss goal, more weight loss initially, increased physical activity, a regular eating pattern, self-monitoring, and flexible control over eating [89, 90].
Depression and other psychiatric disorders can be obstacles to weight loss and weight loss maintenance [89]. There has also been concern raised that dietary restriction may in fact increase binge eating frequency or night eating in individuals who have an eating disorder. Research has not found a consistent association between severe dietary restriction and binge eating or night eating [39, 91]. Binge episodes generally decline during the weight loss attempts [83, 92]. Even with very low calorie diets, binge eaters can lose a similar amount of weight to those without binge eating [83]. It is important to note that binge eating may return when the severe energy restriction or the diet is terminated, but it has not necessarily been found to increase binge eating [83]. While dieting has not been a proven risk factor for BED, weight cycling is associated with binge eating and thus psychological treatments are likely best as first-line interventions to address the eating disorder pathology [10]. Also, in terms of addressing problems associated with obesity in individuals with eating disorders, a focus on normalizing food intake and physical activity with only moderate calorie restrictions is suggested [10, 83].
10.6.2 Psychological Approaches
Cognitive Behavior Therapy (CBT; [72]), Interpersonal Therapy (IPT; [93]), Dialectical Behavior Therapy (DBT; [94]), Motivational Interviewing (MI; [95]), and mindfulness approaches [96] have all been used in treatment studies of BED. CBT is the most studied modality and both CBT and IPT are currently considered empirically validated treatments for BED [21]. Psychological interventions have generally been found to be superior to behavioral interventions in terms of reducing eating pathology and improving psychological functioning, but weight loss has been minimal with these approaches [22]. Grilo et al. [97] found that when substantial improvements in reducing binge eating symptoms occurred within the first 4 weeks of treatment, this rapid response predicted remission at post-treatment in obese patients with BED . Regarding night eating, Berner and Allison [58] reviewed treatments available and found two case studies that suggested that behavioral interventions improved night eating and another study found that progressive muscle relaxation helped to reduce evening appetite [98]. CBT for NES has recently been developed and integrates elements of CBT for insomnia. The preliminary results of a pilot study suggest that a decrease in nocturnal ingestions and NES symptoms were found using this ten-session protocol [28]. While psychological interventions have not been found to reduce weight per se, they are effective first-line interventions for treatment of eating disorder symptoms and may in fact prevent further weight gain by reducing eating disorder pathology [99].
10.7 Psychopharmacology
10.7.1 Stimulants, Antidepressants, and Antiepileptic Medications Have Been Tried Successfully in Treatment of BED
Lisdexamfetamine dimesylate (Vyvanse) is now the first FDA-approved medication to treat moderate to severe binge eating disorder (defined as having at least 3 binge days a week for 2 weeks). The recommended starting dose for Vyvanse is 30 mg to be titrated upwards in 20 mg increments up to 70 mg.
In two studies [100] involving more than 700 people, Vyvanse (50 and 70 mg studied over 12 weeks) decreased the average number of binge days more than a placebo. Vyvanse cut the average number of binge days per week from nearly 5 to less than 1 at the end of the 12-week study.
In another clinical trial [101], half of those taking the 70-mg dose of Vyvanse stopped binge eating during the 4-week period studied, compared to 21 % of those taking placebo. The most common side effects reported were dry mouth, sleeplessness , increased heart rate, a jittery feeling, and anxiety. Caution should be exercised regarding the use of stimulants as they can cause or exacerbate psychosis, mania, or seizures as well as sudden death in people who have existing heart problems. Abuse potential is another consideration.
The efficacy of antidepressant agents in the treatment of BED has been examined. In a meta-analysis of seven RCTs [102], treatment with antidepressants (six with selective serotonin reuptake inhibitors and one with a tricyclic antidepressant) was associated with significantly higher binge eating remission rates (40.5 %) compared with the placebo group (22.2 %). There were no significant differences between the antidepressant and placebo groups in change in mean frequency of binge eating episodes, BMI, or treatment discontinuation. Bupropion [103], however, when compared to placebo did not show any improvement in binge eating frequency but rather weight loss whereas duloxetine demonstrated greater improvement in binge eating, body weight and global measures of improvement when compared to placebo [104].
Two large placebo-controlled RCTs of topiramate in BED found that topiramate treatment was superior to placebo in reducing binge eating frequency, obsessive-compulsive features of binge eating, global severity of illness, body weight, and BMI [105, 106].
Zonisamide was superior to placebo in reducing binge eating frequency, body weight, BMI, measures of global severity, disinhibition of eating, and obsessive-compulsive characteristics of binge eating in a 12-week randomized controlled trial [107].
10.7.2 Antidepressants, Melatonergic Medications, Topiramate , and Light Therapy Might Help in the Treatment of NES
Collectively, limited double-blind controlled studies and case reports suggest that SSRI medications, (particularly sertraline and escitalopram), melatonergic medications, topiramate, and light therapy may be effective for the treatment of NES.
Two double blinded placebo-controlled studies are available studying the impact of antidepressants on NES. Sertraline (mean dose of 126.5 mg over 8 weeks) has improved NES symptoms and quality of life indicators with significant weight loss (2.9+/−3.8 kg) compared to placebo [108]. However, escitalopram (10–20 mg over 12 weeks) did fare better than placebo in terms of NES symptom improvement or weight loss [109].
In open-label studies, there was evidence of improvement in NES symptoms when patients were tried on sertraline [110] and escitalopram [111].
Agomelatine (50 mg/day) was shown in one case report [112] used over 3 months and in a case series of five patients [113] given over 10 weeks to improve NES and depressive symptoms with decrease in average weight change.
Topiramate (75 mg to 125 mg/day) in few case reports [114–116] has shown over 3–9 months trial positive results improving patients’ NES, sleep walking, and nocturnal eating. Two weeks treatment with 10,000 lux of light therapy over 30 min daily has been documented in two case reports to improve NES and depressive symptoms [117, 118].
10.7.3 Bariatric Surgery and Eating Disorders
In terms of weight loss interventions, bariatric surgery is the most effective in that individuals typically lose between 20–40 % of their excess weight [22]. While weight loss surgery rates are increasing, approximately 20–30 % of individuals have difficulty maintaining weight loss post-surgery and will regain weight [119], with postoperative weight regain typically occurring between 18 and 24 months post-surgery [120]. Factors that contribute to weight regain include poor adherence to diet recommendations, a return to unhealthy eating patterns, lack of physical activity, substance abuse, and potential resolution of dumping syndrome [119]. When examining pre-op predictors of weight loss post-surgery, there is little evidence that pre-op disordered eating or other psychiatric disorders predicts post-op weight outcomes [7, 22, 119] and currently, the most robust predictor of postoperative weight loss is postoperative eating behavior and adherence to diet and physical activity recommendations [121]. The longest longitudinal study to date with a 10-year follow-up found that individuals with higher hunger and disinhibition scores 10 years post-surgery were more likely to have gained weight or have lost less than 10 % of baseline weight [122].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






