CHAPTER 9
Dyslipidemias
Roda Plakogiannis, BS, PharmD, BCPS, CLS, FNLA
Coronary heart disease (CHD) is the leading cause of death of both men and women in the United States, and its incidence increases linearly with age. Large population studies demonstrate a clear relation between cholesterol levels and CHD risk, especially in subjects with cholesterol levels more than 200 to 240 mg/dL. Over the last 3 decades, overwhelming evidence has accumulated demonstrating this relation as well as the benefits of lowering serum cholesterol on reducing the risk of CHD. This bidirectional relation led to the establishment of the National Cholesterol Education Program (NCEP). The NCEP Adult Treatment Panel (ATP) III released in 2001 identified low-density lipoprotein cholesterol (LDL-C) as the primary treatment target. The NCEP ATP III report highlighted the importance of measuring the non-high-density lipoprotein cholesterol (non-HDL-C) levels, identified as a secondary target in patients with triglyceride levels exceeding 200 mg/dL, and controlling risk factors of the metabolic syndrome. The non-HDL-C level is calculated by subtracting the patient’s HDL-C from the total cholesterol level, and in return provides an estimate of all the atherogenic particles (intermediate density lipoprotein [IDL], very-low-density lipoprotein [VLDL], LDL-C, and lipoprotein (a) [Lp (a)]). The non-HDL-C goal is then calculated by adding 30 mg/dL to the patient’s determined LDL-C goal. The importance of identifying the metabolic syndrome in patients promotes a dialogue between the health care practitioner and the patient emphasizing lifestyle modification, including weight loss, increase in physical activity, and healthy eating habits. Identifying the metabolic syndrome is paramount as it is associated with insulin resistance, which is one of the underlying roots to the development of type 2 diabetes.
Metabolic syndrome is defined as having three out of the following five criteria:
 Abdominal obesity (waist circumference >40 inches in men, >35 inches in women)
Abdominal obesity (waist circumference >40 inches in men, >35 inches in women)
 Triglycerides ≥150 mg/dL
Triglycerides ≥150 mg/dL
 HDL-C <40 mg/dL in men, <50 mg/dL in women
HDL-C <40 mg/dL in men, <50 mg/dL in women
 Blood pressure ≥130/85 mmHg
Blood pressure ≥130/85 mmHg
 Fasting plasma glucose ≥110 mg/dL
Fasting plasma glucose ≥110 mg/dL
In 2004, an addendum to the ATP III defined optimal LDL-C as <100 mg/dL and <70 mg/dL in patients considered at very high risk for CHD events (Third Report of NCEP, 2002; www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf). The approach of the NCEP is to identify and treat high-risk patients with elevated cholesterol levels (Table 9.1) and to encourage the general public to modify lifestyle habits to reduce cholesterol concentrations with other risk factors for CHD that have been identified (see Chapter 8, Coronary Artery Disease).
The International Atherosclerosis Society (IAS) Position Paper: Global Recommendations for the Management of Dyslipidemia (2013; www.athero.org/IASPositionPaper.asp) was released in July 2013. Its objective is to assist countries in the management of dyslipidemia, especially those without guidelines. The IAS position paper identifies non-HDL-C as a major form of atherogenic cholesterol and highlights targeting either non-HDL-C or LDL-C. The IAS position paper places primary emphasis on lifestyle modification and secondary emphasis on drug therapy.
The American College of Cardiology and American Heart Association (ACC/AHA) in partnership with National Heart, Lung, and Blood Institute (NHLBI) released guidelines for the treatment of cholesterol in November 2013 (Stone et al., 2013; circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437738.63853.7a). The new guidelines do not support titrating lipid-lowering therapies to achieve LDL-C and non-HDL-C goals as previously recommended by NCEP ATP III. These guidelines have raised much debate and at the time of this publication, several societies, such as the National Lipid Association, have not endorsed these guidelines. The National Lipid Association disagrees with the recommendation set in these guidelines to no longer assess for LDL-C goals once the patient is initiated on statin therapy.
Successful management of hyperlipidemic patients requires a thorough understanding of nonpharmacological and pharmacological therapies. Primary care providers must also have an understanding of the multifactorial nature of the process of atherogenesis (see Chapter 8, Coronary Artery Disease). The focus of this chapter is the role of hyperlipidemia in CHD and the current approaches to correcting hyperlipidemia. This chapter will discuss both the NCEP ATP III and 2013 ACC/AHA Blood Cholesterol Guidelines.
Age: male ≥45 y or female ≥55 y
Family history of premature coronary heart disease (definite myocardial infarction or sudden death before 55 y of age in father or other first-degree male relatives, or before 65 y of age in mother or other female first-degree relatives)
Cigarette smoker
Blood pressure ≥140/90 mmHg or taking an antihypertensive medication
HDL-C level <40 mg/dL (if HDL-C ≥60 mg/dL, deduct one positive risk factor because high levels of HDL-C decrease the risk of coronary heart disease)
HDLC, high-density lipoprotein cholesterol; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III.
Source: Third Report of NCEP (2002).
 ANATOMY, PHYSIOLOGY, AND PATHOLOGY
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Lipid Metabolism and Pathophysiology
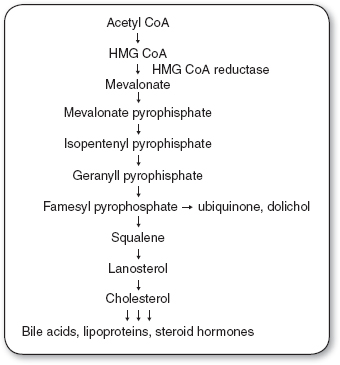
Cholesterol is a water-insoluble molecule that is essential for cell membrane formation and the synthesis of steroid hormones and bile acids. Cells derive cholesterol from intracellular synthesis or extraction from the systemic circulation. Daily cholesterol intake ranges from 300 to 500 mg, with approximately 50% being absorbed (Grundy, 1978). Body synthesis of cholesterol ranges from 500 to 1,000 mg/dL (Grundy & Ahrens, 1966, 1969) Therefore, approximately 25% of the body’s total cholesterol source is from dietary intake and 75% is synthesized in vivo. Cellular synthesis of cholesterol takes place through a series of biochemical steps involving a number of enzymes (Figure 9.1). An important class of drugs, the hydroxymethylglutaryl coenzyme A (HMG CoA) reductase inhibitors, also referred to as the statins, disrupts this process; these drugs are commonly used in patients with elevated cholesterol levels.
Cholesterol and triglycerides in the diet enter into the exogenous pathway of lipid transport. Cholesterol in the intestine must be solubilized before it can be absorbed, and absorption is incomplete because cholesterol is highly insoluble in aqueous solutions (Grundy, 1996). In the intestine, cholesterol is in the unesterified (free) form and becomes esterified with a fatty acid when it enters the intestinal mucosa. Triglycerides are reformulated from absorbed fatty acids and monoglycerides are incorporated with cholesterol esters into chylomicrons. These lipoproteins eventually reach the systemic circulation and undergo partial catabolism to chylomicron remnants carrying newly absorbed cholesterol into the liver (Grundy, 1996).
Hepatic cholesterol comes from newly absorbed cholesterol, from the uptake of serum lipoproteins, and via hepatic synthesis. In the liver, cholesterol can be incorporated into lipoproteins that are secreted into the plasma; their density is determined by their relative content of protein and lipid. Hepatic cholesterol synthesis (endogenous pathway) is regulated by the quantity of cholesterol in the liver. Bile acid synthesis is governed by the quantity of bile acids returning to the liver via enterohepatic circulation (Grundy, 1996). The primary route of cholesterol excretion is into the bile as cholesterol or as bile acids. About one third of the cholesterol synthesized or absorbed is converted into bile acids, which can be reabsorbed into the portal circulation and return to the liver (Grundy & Ahrens, 1966, 1969).
Lipid disorders can result from a dysfunction in any one of the numerous steps involved in the process of lipid metabolism. Table 9.2 categorizes the more common lipid disorders.
Lipoproteins
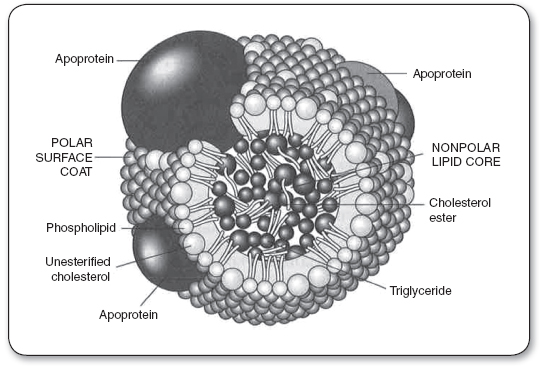
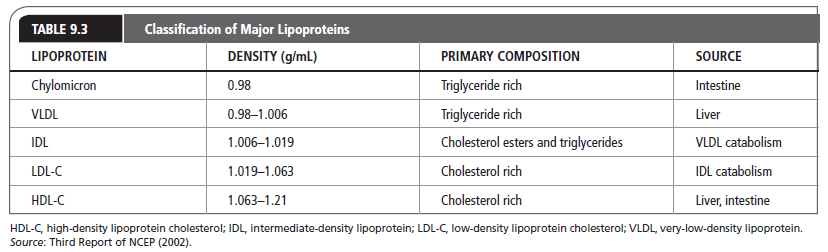
Lipids are present in the plasma as circulating lipoproteins that transport cholesterol and triglycerides. The basic structure of a typical lipoprotein is shown in Figure 9.2. These lipoproteins are divided into classes, typically based on their density and composition. The major lipoproteins include chylomicrons, VLDL, IDL or remnant particles, LDL-C, and HDL-C (Table 9.3). LDL-C accounts for approximately 60% to 70% of total serum cholesterol, HDL-C about 20% to 30%, and VLDL about 10% to 15%. In addition, each lipoprotein particle contains proteins on its outer surface called apolipoproteins. These proteins have several functions, such as activating enzyme systems, providing structure to the lipoprotein, and binding with cell receptors.
Apolipoproteins
Four major classes of apolipoproteins (apo) exist: apo B, apo A, apo C, and apo E (Table 9.4). Patients with abnormal metabolism of apolipoproteins may be at an increased risk of atherosclerosis even if they have normal cholesterol levels. Evidence suggests that apo B measurements may be better predictors in estimating cardiovascular risk than the traditional cholesterol panel (Barter et al., 2006), thus suggesting that apo B measurement be included in the traditional cholesterol panel (Di Angelantonio et al., 2009). Therefore, many health care providers believe that apolipoprotein levels, specifically apo B, should be used to evaluate hyperlipidemic patients especially in patients with the metabolic syndrome or type 2 diabetes.
Common Lipid Disorders |
DISORDER | DEFECT | EFFECT |
Defective Clearance | ||
Familial hypercholesterolemia (heterozygous; type lla) | LDL-C receptors | ↑ LDL-C |
Dysbetalipoproteinemia (type III) | Apo E | ↑ IDL, remnant VLDL |
Familial defective apo B-100 | Apo B | ↑ LDL-C |
Polygenic hypercholesterolemia | LDL-C receptor activity | ↑ LDL-C |
Familial lipoprotein lipase deficiency (type V) | Lipoprotein lipase | ↑ Chylomicrons triglycerides |
Increased Production | ||
Familial hypercholesterolemia (heterozygous) | ↑ Apo B | ↑ Apo B |
Familial combined hyperlipidemia (type IIb) | ↑ Apo B and VLDL | ↑ Cholesterol and/or ↑ triglycerides |
Hypoalphalipoproteinemia | ↑ HDL-C catabolism (low HDL-C) | ↓ HDL-C |
Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; IDL, intermediate-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; VLDL, very-low-density lipoprotein.
Chylomicrons
Synthesized in the intestine, chylomicrons are large triglyceride-rich particles with apo B-48, apo A, apo C, and apo E. In the peripheral circulation, chylomicrons interact with lipoprotein lipase (LPL) on the vascular endothelium, which hydrolyzes the triglycerides into free fatty acids and monoglycerides, which are then absorbed by muscle and adipose tissues. The resultant product is a cholesterol-rich chylomicron remnant that is taken up by the liver. Patients with a LPL deficiency or apo C-II deficiency (C-II is the LPL activating factor) will develop hypertriglyceridemia.
Classification of Major Apolipoproteins |
APOLIPOPROTEIN | LIPOPROTEINS | SOURCES |
Apolipoprotein Bs | ||
B-48 | Chylomicrons | Intestine |
B-100 | VLDL, LDL-C | Liver |
Apolipoprotein As | ||
A-I | Chylomicrons, HDL-C | Liver, intestine |
A-II | Chylomicrons, HDL-C | Liver |
A-IV | Chylomicrons, HDL-C | Intestine |
Apolipoprotein Cs | ||
C-I | Chylomicrons, VLDL, HDL-C | Liver |
C-II | Chylomicrons, VLDL, HDL-C | Liver |
C-III | Chylomicrons, VLDL, HDL-C | Liver |
Other | ||
E2–E4 | Chylomicrons, VLDL, HDL-C | Liver |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL, very-low-density lipoprotein.
Source: Third Report of NCEP (2002).
Newly absorbed cholesterol thus passes into the liver in chylomicron remnants. Fatty acids of chylomicron triglycerides are released into the peripheral circulation and can be used by muscles for energy, adipose tissue, and the liver. After a fatty meal, chylomicron levels are elevated, and therefore triglycerides are high. However, after a 12-to 24-hour fast, chylomicrons will have cleared from the blood. Fasting triglyceride concentrations reflect hepatic production and that which is carried by VLDL and other remnant particles. For this reason, patients are required to fast before obtaining a lipoprotein profile.
Very-Low-Density Lipoproteins
Triglyceride-rich VLDL particles are produced by the liver and contain apo B-100 and apo E on their surface. The nascent VLDL particles circulate and acquire cholesterol esters and apolipoproteins (including apo C), developing into mature VLDLs. The B and E proteins are ligands for LDL-C receptors, and a defect in these receptors results in increased levels of cholesterol. Apo C-II activates LPL, which interacts with VLDL as it does with chylomicrons. Fatty acids are released, producing remnant VLDL and IDL. These remnants are eventually metabolized by the liver or converted to LDL-C. Most of the triglycerides are removed and replaced with cholesterol esters. Deficiencies of apo C-II result in faulty triglyceride metabolism and hypertriglyceridemia.
Low-Density Lipoproteins
The LDLs are the major cholesterol transport lipoproteins in the plasma. They contain cholesterol ester in their core and only apo B-100 on their surface. LDL-C is derived from VLDL catabolism and cellular synthesis. LDL-C is cleared by the liver and extrahepatic tissues, which recognize the apo B-100 and bind and internalize the particle, which is then degraded. Excess circulating LDL-C may undergo oxidative modification enhancing macrophage uptake and foam cell formation resulting in deposition of cholesterol outside the cell, causing the formation of atherogenic plaques in the vascular endothelium. Because LDL-C contains 60% to 70% of the total blood cholesterol and there is a direct relation between the LDL-C in the systemic circulation and atherosclerosis, LDL-C is the primary target of cholesterol-lowering therapies, in accordance with NCEP ATP III guidelines.
The smallest of the lipoprotein particles, HDL-C appears to transport cholesterol from peripheral cells to the liver. The liver and the gut secrete nascent HDL-C that consist of apo A and phospholipids. These particles acquire unesterified cholesterol that becomes esterified by lecithin-cholesterol acyltransferase, forming HDL-3 particles. HDL-3 acquires more cholesterol, forming HDL-2, which in turn may be converted back to HDL-3 by hepatic lipase and by transferring cholesterol esters to the liver, LDL-C, and VLDL. The transfer of cholesterol from HDL particles to lipoproteins is catalyzed by the enzyme cholesterol ester transfer protein. This complex HDL cycle is a critical part of the reverse cholesterol transport process, where cholesterol is returned to the liver for excretion.
In contrast to LDL-C, high HDL-C concentrations are desirable because they provide protective effects against the atherogenic process. Because HDL particles contain apo A-I, which activates lecithin-cholesterol acyltransferase, levels of apo A-I have a strong inverse correlation with CHD. Patients with abnormally low HDL-C levels (<40 mg/dL) are at an increased risk of CHD, presumably because of the decreased ability to remove circulating cholesterol. In general, for every 1% decrease in HDL-C, there is a 2% to 3% increase in CHD (Chapman, Assmann, Fruchart, Shepherd, & Sirtori, 2004). In contrast, an elevated HDL-C level (≥60 mg/dL) is considered a negative risk factor for CHD.
Low-Density-Lipoprotein Receptors
Cholesterol uptake by hepatic and peripheral cells is accomplished by the binding of apolipoproteins found on circulating lipoproteins to LDL-C receptors on cell surfaces. LDL-C receptor synthesis occurs when there are low concentrations of intracellular cholesterol. These receptors are capable of binding with lipoproteins containing apo E or apo B-100. Once bound, these lipoproteins are degraded. Drugs capable of reducing the intracellular synthesis of cholesterol can upregulate LDL-C receptors, thus enhancing removal of cholesterol from the systemic circulation.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Rationale for Therapy of Dyslipidemia
Epidemiological studies have greatly contributed to our understanding of CHD in patients with high blood cholesterol levels. A direct relation between total cholesterol and LDL-C levels in the blood and death and disability from CHD has been demonstrated consistently. One of the most notable studies is the Framingham Heart Study (Kannel, Castelli, Gordon, & McNamara, 1971). In this ongoing study, the population of Framingham, Massachusetts has been followed for more than 65 years, documenting that cardiovascular risk factors are related to CHD rates where serum cholesterol levels are correlated with CHD rates (Mahmood, Levy, Vasan, & Wang, 2013). In addition, long-term survival was inversely related to total cholesterol level at entry into the study (Anderson, Castelli, & Levy, 1987).
Another investigation, the Multiple Risk Factor Intervention Trial, involved more than 360,000 men in the United States (Stamler, Wentworth, & Neaton, 1986). The study participants were screened and followed for 6 years for CHD mortality. This trial showed a positive curvilinear correlation between initial cholesterol levels and subsequent CHD mortality, further emphasizing the relation between high cholesterol levels and CHD.
Individual lipoprotein fractions and CHD rates have similarly been established. The Framingham Heart Study (Kannel, Castelli, & Gordon, 1979) revealed the association between risk factors and CHD: a high LDL-C level was found to be more predictive of CHD than the total cholesterol level. The significance of this relation is evident in the NCEP’s recommendations, in which LDL-C is the primary target of therapy in patients with hyperlipidemia. This same study (Gordon, Castelli, Hjortland, Kannel, & Dawber, 1977; Kannel et al., 1979) has also provided valuable data about the negative relation between HDL-C level and CHD.
Serum triglyceride concentrations are also correlated with CHD risk (Austin, 1991; Sarwar et al., 2007). The Framingham Heart Study (Castelli, 1986) showed that the VLDL level (reflecting serum triglycerides) was a predictor of CHD. In patients with hypertriglyceridemia, there is often an accompanying increase in other atherogenic lipoproteins and a decreased HDL-C level. Therefore, an elevation in triglycerides may result in an increase of other lipoproteins that promote CHD. When serum triglycerides exceed 1,000 mg/dL, the most immediate danger is acute pancreatitis. In patients with diabetes mellitus, reduction of glucose levels may significantly lower triglyceride levels.
Etiology of Dyslipidemia
The most severe forms of dyslipidemia occur in patients with a defect in lipid metabolism or transport (see Table 9.2). Persons with these hereditary diseases or primary causes of hyperlipidemia typically require pharmacotherapy in conjunction with lifestyle modifications to correct or normalize their condition.
Secondary causes of hyperlipidemia can be attributed to lifestyle and underlying disease states (Table 9.5). In addition, many medications can have a negative effect on the lipid profile (Table 9.6). Many of these medications are used to treat comorbid disease states such as hypertension, heart failure, and angina. Primary care providers should evaluate the hyperlipidemic patient’s current medications and identify any drugs that may be contributing to the dyslipidemic state. In some cases, a suspected medication can be replaced by one that has a neutral effect on lipids or one that has a beneficial effect. Angiotensin-converting enzyme (ACE) inhibitors and calcium channel blockers have a neutral effect on lipids; postsynaptic alpha-1 receptor blockers (e.g., prazosin, terazosin, doxazosin) have favorable lipoprotein effects (Stone, 1994).
Secondary Causes of Hyperlipidemia (Disease Induced) |
Acromegaly Diabetes mellitus Hypothyroidism Chronic renal failure Hepatic disease Cushing syndrome Polycystic ovarian syndrome | Glycogen storage disease Nephrotic syndrome Anorexia nervosa Lipodystrophy Systemic lupus erythematosus Human immunodeficiency virus Pregnancy |
Source: Third Report of NCEP (2002).
Secondary Causes of Hyperlipidemia (Drug Induced) |
MEDICATION CLASS | COMMENTS |
Diuretics | Thiazides: ↑LDL-C and triglycerides, with the exception of indapamide Loop: ↓ HDL-C |
Beta-blockers | Selective and nonselective: ↑ triglycerides and ↓ HDL-C, with the exception of those that are alpha-blocking or with ISA activity |
Cyclosporine | ↑ LDL-C and triglycerides |
Anabolic steroids | ↑ LDL-C and ↓ HDL-C |
Isotretinoin | ↑ Cholesterol and triglycerides, ↓ HDL-C |
Glucocorticoids | ↑ HDL-C and triglycerides |
Alcohol | ↑ Triglycerides |
Protease inhibitors | ↑ Triglycerides |
Bile acid sequestrants | ↑ Triglycerides (avoid use in when triglycerides exceed 500 mg/dL) |
Oral contraceptives | ↑ Cholesterol and triglycerides, ↑ HDL-C: because of progestins; estrogen alone is protective; effects will vary between available formulations |
Atypical antipsychotics | ↑ Triglycerides |
Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; ISA, intrinsic sympathomimetic activity; LDL-C, low-density lipoprotein cholesterol.
Source: Stone (1994).
A person’s dietary habits and lifestyle have a significant effect on the lipid profile and body weight, which can be positively correlated with LDL-C and triglyceride levels and negatively correlated with HDL-C levels. Patients who are overweight will benefit from weight loss, as seen by reduced LDL-C and triglyceride levels and an increased HDL-C level. Modifications in diet can result in weight loss and improvements in the lipoprotein profile. For these reasons, lifestyle modifications including dietary therapy are considered the foundation of therapy.
 DIAGNOSTIC CRITERIA
DIAGNOSTIC CRITERIA
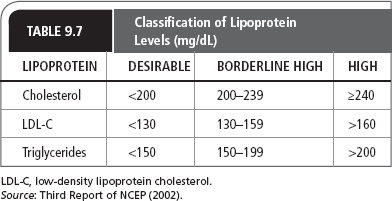
Normal and abnormal lipoprotein levels according to the NCEP ATP III guidelines (2002) are listed in Table 9.7. According to the 2013 ACC/AHA guidelines, decisions on treatment are based on a patient’s 10-year risk of developing cardiovascular disease. Four groups of patients have been identified to benefit most from statin therapy:
 Adults, 21 years or older, with a history of cardiovascular disease
Adults, 21 years or older, with a history of cardiovascular disease
 Adults with an LDL-C ≥190 mg/dL
Adults with an LDL-C ≥190 mg/dL
 Patients with diabetes, aged 40 to 75 years, with an LDL-C between 70 and 189 mg/dL
Patients with diabetes, aged 40 to 75 years, with an LDL-C between 70 and 189 mg/dL
 Patients, aged 40 to 75 years with an LDL-C between 70 and 189 mg/dL, with an estimated 10-year risk of CHD ≥7.5% (Stone et al., 2013)
Patients, aged 40 to 75 years with an LDL-C between 70 and 189 mg/dL, with an estimated 10-year risk of CHD ≥7.5% (Stone et al., 2013)
 HISTORY AND PHYSICAL EXAMINATION
HISTORY AND PHYSICAL EXAMINATION
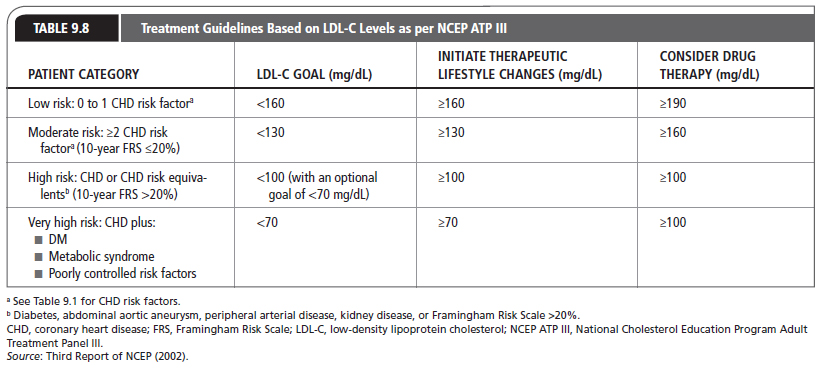
Patients should be thoroughly assessed with a complete evaluation (e.g., complete history, physical examination, and basic laboratory tests; see Chapter 8, Coronary Artery Disease). CHD risk equivalents (e.g., diabetes, Framingham Risk Scale [FRS] >20%, abdominal aortic aneurysm, peripheral arterial disease, or kidney disease) should be identified. Patients with CHD risk equivalents are considered at high risk of developing CHD and, according to NCEP ATP III guidelines, their target LDL-C level is <100 mg/dL (Table 9.8). As outlined in the addendum to the NCEP ATP III guidelines (Grundy et al., 2004; http://circ.ahajournals.org/content/110/2/227.full), targeting lower LDL-C (<70 mg/dL) levels in the very high-risk patient, with established CHD and multiple risk factors, demonstrated added benefit.
The FRS is calculated to determine a patient’s 10-year CHD risk when two or more CHD risk equivalents are present. The FRS assessment tool is available on the NHLBI website (cvdrisk.nhlbi.nih.gov/calculator.asp). If a patient is found to have hyperlipidemia that cannot be explained by secondary causes, genetic factors may be the cause of the elevated lipids.
The IAS simplifies LDL-C and non-HDL-C goals. Target lipoproteins for primary prevention and secondary prevention include LDL-C <100 mg/dL or non-HDL-C <130 mg/dL, and LDL-C <70 mg/dL or non-HDL-C <100 mg/dL, respectively. Secondary prevention includes patients with established CHD, stroke, peripheral arterial disease, carotid artery disease, and other forms of atherosclerotic vascular disease (International Atherosclerosis Society, 2013).
The 2013 ACC/AHA guidelines have moved away from targeting lipoproteins and recommend moderate to intensive statin therapy targeting four types of individuals (adults, aged 21 years or older, with a history of cardiovascular disease; those with LDL-C ≥190 mg/dL; diabetics aged 40–75 years with an LDL-C between 70 and 189 mg/dL; or patients aged 40–75 years with an LDL-C between 70 and 189 mg/dL, with an estimated 10-year risk of CHD ≥7.5%). For these groups, atherosclerotic CHD risk reduction outweighs the risk of potential statin-associated adverse event(s). The cardiovascular risk calculator is available on the AHA website (http://my.americanheart.org/cvriskcalculator).
 DIAGNOSTIC STUDIES
DIAGNOSTIC STUDIES
According to NCEP ATP III guidelines, a fasting lipid profile should be measured in all adults aged 20 years and older at least once every 5 years. The lipoprotein profile includes total serum cholesterol, total triglycerides, LDL-C, and HDL-C (Table 9.7). The LDL-C can be estimated by using the Friedewald equation (which is often automatically calculated by the laboratory):
LDL-C = (total cholesterol) – (triglycerides/5) – HDL-C
In the above equation, the term “triglycerides/5” represents the VLDL. The Friedewald equation is valid when the triglyceride levels are below 400 mg/dL and fasting for 9 to 12 hours is recommended for an accurate LDL-C level. Secondary prevention in adults requires a lipoprotein analysis and classification based on the LDL-C levels (Table 9.7).
According to the 2013 ACC/AHA guidelines, for patients, aged 40 to 75 years without clinical cardiovascular disease or diabetes and not currently on statin therapy, the decision to treat is based on 10-year risk of developing cardiovascular disease; the risk should be recalculated every 4 to 6 years.
 TREATMENT OPTIONS, EXPECTED OUTCOMES, AND COMPREHENSIVE MANAGEMENT
TREATMENT OPTIONS, EXPECTED OUTCOMES, AND COMPREHENSIVE MANAGEMENT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree