INTRODUCTION
Dyshemoglobinemias are disorders in which the hemoglobin molecule is functionally altered and prevented from carrying oxygen. The most clinically relevant dyshemoglobinemias are carboxyhemoglobin, methemoglobin, and sulfhemoglobin.1 Carboxyhemoglobin is created during carbon monoxide exposure and, because of its unique importance and prevalence, is usually considered an environmental emergency (see chapter 222, “Carbon Monoxide”).
METHEMOGLOBINEMIA
The iron moiety within deoxyhemoglobin normally exists in the ferrous (bivalent or Fe2+) state. Ferrous iron avidly interacts with compounds seeking electrons, such as oxygen or other oxidizing agent, and in the process is oxidized to the ferric (trivalent or Fe3+) state. Hemoglobin in the ferric form is unable to bind oxygen for transport and is termed methemoglobin. Under normal circumstances, <1% to 2% of circulating hemoglobin exists as methemoglobin; higher concentrations define the condition of methemoglobinemia.
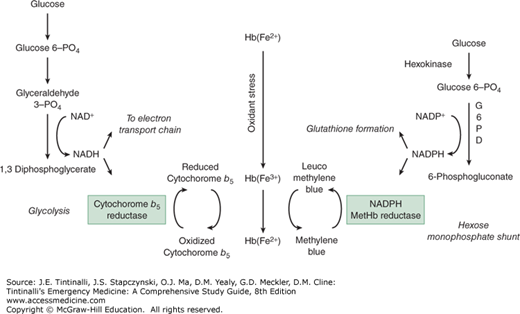
Methemoglobin accumulation is enzymatically prevented by the rapid reduction of the ferric iron back to the ferrous form. Cytochrome b5 reductase is primarily responsible for this reduction, in which reduced nicotinamide adenine dinucleotide donates its electrons to cytochrome b5, which subsequently reduces methemoglobin to hemoglobin (Figure 207-1). This pathway is responsible for reducing nearly 95% of methemoglobin produced under typical circumstances. Methemoglobinemia occurs when this enzymatic reduction is overwhelmed by an exogenous oxidant stress, such as a drug or chemical agent (Table 207-1).
| Oxidant | Comments |
|---|---|
| Analgesics | |
| Phenazopyridine | Commonly reported |
| Phenacetin | Rarely used |
| Antimicrobials | |
| Antimalarials | Common |
| Dapsone | Hydroxylamine metabolite formation is inhibited by cimetidine |
| Local anesthetics | |
| Benzocaine | Most commonly reported of the local anesthetics |
| Lidocaine | Rare |
| Prilocaine | Common in topical anesthetics |
| Dibucaine | Rare |
| Nitrates/nitrites | |
| Amyl nitrite | Cyanide antidote kit and used to enhance sexual encounters |
| Isobutyl nitrite | Used to enhance sexual encounters |
| Sodium nitrite | Cyanide antidote kit |
| Ammonium nitrate | Cold packs |
| Silver nitrate | Excessive topical use |
| Well water | Problem in infants, due to nitrate fertilizer runoff |
| Nitroglycerin | Rare |
| Sulfonamides | |
| Sulfamethoxazole | Uncommon |
FIGURE 207-1.
Methemoglobin formation and mechanism of action of methylene blue. G6PD = glucose-6-phosphate dehydrogenase; Hb(Fe2+) = hemoglobin; Hb(Fe3+) = methemoglobin; NAD+ = oxidized nicotinamide adenine dinucleotide; NADH = reduced form of nicotinamide adenine dinucleotide; NADP+ = nicotinamide adenine dinucleotide phosphate; NADPH = reduced form of nicotinamide adenine dinucleotide phosphate; PO4 = phosphate.
Methemoglobin can also be reduced by a second enzymatic pathway using the reduced form of nicotinamide adenine dinucleotide phosphate (or NADPH) and NADPH-methemoglobin reductase.2 This pathway is normally of minimal importance and is responsible for less than 5% of total reduction under typical circumstances. However, this enzyme and pathway are crucial for the antidotal effect of methylene blue (Figure 207-1).
The limited role for NADPH partially explains why patients with glucose-6-phosphate dehydrogenase deficiency with a resultant deficiency in NADPH are not at increased risk of developing methemoglobinemia, although they are at risk of developing hemolysis following exposure to an oxidant stress. To a very limited extent, nonenzymatic reduction systems, such as vitamin C and glutathione, may participate in the reduction of methemoglobin to hemoglobin.
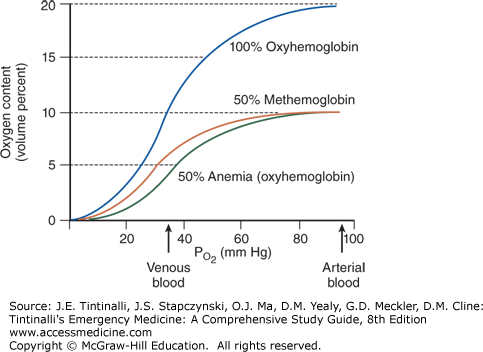
The primary clinical effect of methemoglobin is to reduce the oxygen content of the blood. Because hemoglobin-bound oxygen accounts for the vast majority of an individual’s oxygen-carrying capacity, as the methemoglobin concentration rises, oxygen-carrying capacity to the tissues falls. Patients with methemoglobinemia are often more symptomatic than patients who suffer from a simple anemia that produces an equivalent reduction in oxygen-carrying capacity. This is caused by a leftward shift in the oxyhemoglobin dissociation curve, the consequence of which is a reduced release of oxygen from the erythrocyte to the tissue at a given partial pressure of oxygen (Figure 207-2).2
The oxyhemoglobin dissociation curve of blood with a 50% reduction in erythrocytes (i.e., anemia) follows a curve similar to that of nonanemic blood; although the oxygen content is lower, unbinding of half of the oxygen (50% oxygen saturation) occurs at the same PO2. With 50% methemoglobin, the leftward shift of the oxyhemoglobin dissociation curve means that hemoglobin is less willing to give up its oxygen, so that tissue hypoxia is more severe than in those with a 50% anemia.
Drugs in conventional doses rarely produce clinically significant methemoglobinemia, although subclinical methemoglobinemia may go unrecognized (Table 207-1). Benzocaine is the local anesthetic most commonly associated with methemoglobinemia.3,4,5 Methemoglobin induction with sodium nitrite is a therapeutic goal in the management of patients suffering from cyanide poisoning (see chapter 204, “Industrial Toxins”). Certain compounds, particularly dapsone,6 require metabolism to the “active” oxidant, and there may be substantial delay until toxicity is evident. Occupational methemoglobinemia usually involves exposure to aromatic compounds, primarily amino- and nitro-substituted benzenes.7 Routes of absorption are typically dermal or inhalational due to the high lipophilicity and volatility of these compounds, respectively.
Neonates and infants are more susceptible to methemoglobin accumulation because of undeveloped methemoglobin reduction mechanisms. This accounts for the relatively common development of methemoglobinemia in infants given certain nitrogenous vegetables (e.g., spinach) or well water that contains high nitrate levels (generally from fertilizer use). Bacteria within the GI flora convert nitrate to the nitrite form, which is a more potent oxidant. Another common cause of acquired infantile methemoglobinemia is gastroenteritis, which presumably is caused by an increased oxidant burden originating in the GI tract.8
Hereditary methemoglobinemia results from either an enzymatic deficiency (i.e., cytochrome b5 reductase) or from the presence of an amino acid substitution within the hemoglobin molecule itself, termed hemoglobin M.1 Patients with cytochrome b5 reductase deficiency develop methemoglobin levels of 20% to 40%. Cyanosis in these individuals begins at birth, but they remain asymptomatic and develop normally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree