Do Not Use Benzocaine Spray: It Increases the Risk of Methemoglobinemia
Kelly Grogan MD
Topical anesthetics are commonly used for procedures including endotracheal intubation, endoscopy, bronchoscopy, laryngoscopy, orogastric tube placement, dental extractions, and office gynecological procedures. Topical anesthetics are also widely available in several over-the-counter medications such as teething gels and throat lozenges. However, one topical anesthetic whose use should be avoided is benzocaine spray (aka Hurricaine spray) due to the risk of methemoglobinemia. Benzocaine-induced methemoglobinemia is an uncommon occurrence in clinical practice; however, knowledge of this potentially life-threatening condition is necessary to those practitioners who perform procedures requiring local, topical anesthesia.
Physiology and Pathophysiology
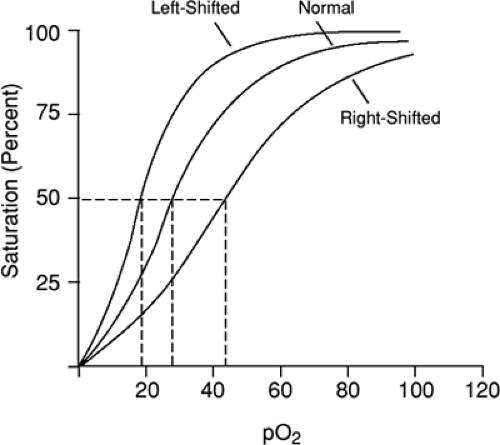
Methemoglobinemia occurs when an imbalance due to either increased methemoglobin production or decreased methemoglobin reduction is present. Normal hemoglobin contains an iron molecule that exists in the divalent ferrous state (Fe2+). Methemoglobin results from the conversion of the iron ferrous ion (Fe2+) to the oxidized ferric (Fe3+) state. The ferric hemes of methemoglobin are unable to bind oxygen. In addition, the oxygen affinity of any accompanying ferrous hemes in the hemoglobin tetramer is increased. This results in a “left shift” in the oxygen dissociation curve and oxygen delivery to the tissues is impaired (Fig. 49.1). Therefore, the patient with increased concentrations of methemoglobin has a functional anemia as the circulating methemoglobin-containing molecules are unable to carry oxygen and deliver it to the tissues.
Auto-oxidation of hemoglobin to methemoglobin occurs spontaneously at a slow rate in normal individuals, converting 0.5% to 3% of the available hemoglobin to methemoglobin per day. The only physiologically important pathway for reducing methemoglobin back to hemoglobin is the nicotinamide adenine dinucleotide (NADH) reductase-dependent reaction catalyzed by cytochrome b5 reductase. An alternative pathway is an enzyme utilizing nicotine adenine dinucleotide phosphate (NADPH) generated by glucose-6-phosphate
dehydrogenase (G6PD). There is normally no electron carrier present in red blood cells to interact with NADPH methemoglobin reductase, making this reaction a minor player in the conversion of methemoglobin to hemoglobin. However, its activity is markedly enhanced by electron acceptors or redox dyes, such as methylene blue.
dehydrogenase (G6PD). There is normally no electron carrier present in red blood cells to interact with NADPH methemoglobin reductase, making this reaction a minor player in the conversion of methemoglobin to hemoglobin. However, its activity is markedly enhanced by electron acceptors or redox dyes, such as methylene blue.
Most cases of methemoglobinemia are acquired, resulting from increased methemoglobin formation by various exogenous agents. The mechanism involved in the formation of methemoglobin is not clear but appears to be the direct or indirect oxidation of hemoglobin to a degree that overwhelms the capacity of the reductive pathway. Local anesthetics have been implicated as a cause of methemoglobinemia for more than 50 years. Although almost all topical anesthetics have been associated, benzocaine is the most commonly implicated agent. Of the cases reported, more than half involved infants and the elderly. Cases have been reported after application to the pharyngeal mucosa, rectal mucosa, vaginal mucosa, and skin. The majority of cases of hereditary methemoglobinemia are due to a deficiency of NADPH methemoglobin reductase. This autosomal recessive disease is most common in the Inuit population and in Alaskan Native Americans. Hemoglobin M is another form of congenital methemoglobinemia characterized by an abnormal hemoglobin molecule.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree