KEY POINTS
Immersion and diving produce physiologic effects from increased hydrostatic pressure and its effects on the physical behavior of gases.
Diving with compressed air (or other breathing mixtures) causes the body to take up extra nitrogen or other inert gases in proportion to the pressure change. During ascent, these dissolved gases must leave gradually through the lungs to avoid decompression sickness (DCS).
DCS is caused by gases leaving solution and forming bubbles in body tissues, leading to musculoskeletal pain (bends) or neurologic symptoms due to direct vascular damage, ischemia, edema, and inflammation.
DCS usually manifests within minutes to hours of surfacing and is emergently treated with high-flow O2 and the administration of fluids. Oxygen recompression in a hyperbaric chamber is the definitive treatment for signs and symptoms and for the prevention of recurrences, even if delayed 1 or 2 days.
Arterial gas embolism (AGE) is the result of overexpansion of the lungs during ascent from diving with compressed gas. It is usually associated with rapid ascent and CNS symptoms and after drowning, is the second leading cause of fatalities in recreational diving. AGE is a medical emergency that also occasionally occurs in the hospital setting.
Drowning is defined by asphyxia in water, and is usually associated with aspiration of water. In survivors, the primary injuries are to the brain, heart, lungs, and kidneys. Drowning is common in children, and in young adult males, and is often associated with drug or alcohol ingestion.
Drowning may be accompanied by traumatic injuries and complicated by acute respiratory distress syndrome (ARDS), often of late onset and often aggravated by aspiration of gastric contents or other foreign debris.
Drowning may be complicated by pneumonia (or sepsis) caused by unusual pathogens present in contaminated water. However, the prophylactic administration of antibiotics is not recommended.
Water sports are enjoyed by millions of people of all ages throughout the world, but the water environment is deceptively hazardous, and swimmers, divers, and boaters display various degrees of skill, experience, and judgment. Too often, inexperienced swimmers or divers venture into perilous conditions with deadly results. In many cases, they have ignored their physical limitations or impaired their faculties with alcohol or other drugs. In some situations, such as with young children, the encounter with water is unsupervised or unexpected. The exact numbers of such aquatic incidents worldwide and their effect on health care systems are difficult to estimate, but according to the Global Burden of Disease the overall death rate by drowning is around 8.4/100,000 people. This converts to more than half a million deaths per year and probably several times that number of drowning episodes. Many victims survive the incident only to die hours or days later in the hospital. In the United States and other westernized nations, there are also several million active sports divers, and there are several thousand diving accidents each year. Thus, the consequences and management of victims of drowning incidents and recreational diving accidents must be familiar to the intensive care specialist.
THE PHYSICS OF UNDERWATER ENVIRONMENTS
The physiologic changes produced by the underwater environment are a result of the direct effects of increased hydrostatic pressure and its effects on the physical behavior of gases. Pressure is measured in units of force per area, which can be expressed in several convenient forms (Table 132-1). At sea level, the pressure of the atmospheric column is approximately 760 mm Hg (14.7 lb/in2). Underwater, the pressure of the water column must be added to the atmospheric pressure to obtain total pressure, usually expressed in atmospheres absolute (ATA). The water pressure is directly proportional to the depth; for instance, a seawater column 33 ft deep (fsw) exerts a pressure equivalent to 1 atmosphere of air at sea level. Thus, a diver at 33 fsw is exposed to a total pressure of 2 ATA.
In diving on compressed air, the diver must inhale at an inspired gas pressure that is very close to the absolute pressure surrounding the body. This means the lungs (or other gas-filled cavities) must be filled with a larger number of gas molecules in order to maintain a constant volume at a given temperature. The relation of pressure (P), volume (V), temperature (T) to number of moles of gas (n) is described by the ideal gas law:
where R is the universal gas constant. The ideal gas law gives rise to the special gas laws important in diving. The three special gas laws relevant to diving are shown in Table 132-1 where one of the three variables is held constant. The most important of these is Boyle’s law, which accounts for the change in volume with a change in pressure and explains the need to equilibrate pressure inside gas-containing spaces in the body, such as the middle ear spaces and lungs, to avoid barotrauma of descent (eg, ear squeeze) or ascent (eg, gas embolism).
Air is composed of mixtures of different molecules in which the total pressure is equal to the sum of the partial pressures of each gas. This reflects Dalton’s law of partial pressures, which states that each gas in a mixture behaves as though it alone occupies the entire space. The uptake of gases by tissue is determined primarily by the diffusion of gas from the alveolar spaces into blood and by transport of gas to tissues by the circulation (perfusion). The amount of a gas dissolved in liquid at any temperature, such as blood or tissues of the body at 37°C (98.6°F), is also proportional to its partial pressure (Henry’s law). The gas concentration in tissue at equilibrium is related to the partial pressure of the gas multiplied by its solubility coefficient. The physiologic effects of diving, such as nitrogen narcosis and the requirement for decompression, and decompression illnesses such as decompression sickness (DCS) and arterial gas embolism (AGE) generally correlate with the partial pressure of the gas in the body tissues.
IMMERSION AND BREATH-HOLD DIVING
Water immersion produces three main physiologic effects: a decrease in thoracic gas volume, an increase in cardiac output, and a diuresis.1 The blood vessels outside the thorax are supported by water, and the upright body is exposed vertically to a hydrostatic pressure gradient that compresses the abdomen relative to the thorax, thereby causing negative pressure breathing (approximately −20 cm H2O). The diaphragm is displaced upward, which decreases thoracic gas volume and expiratory reserve volume. The pressure gradient across the diaphragm, coupled to a hydrostatic stiffening of the venous capacitance in the legs, increases the thoracic blood volume by about 20%, including the heart. Arterial vasoconstriction may further increase the central blood volume if the water temperature is below the thermoneutral point (∼34°C, 93.2°F).
The cardiovascular distention accompanying immersion activates mechanoreceptors that normally respond to hypervolemia. This apparent hypervolemia is sensed in the hypothalamus via vagal afferents and leads to an immersion response consisting of diuresis and natriuresis. Their profiles suggest that they operate by different mechanisms because peak diuresis occurs rapidly (1-2 hours) while peak natriuresis occurs slowly (4-5 hours). Immersion diuresis but not natriuresis can be prevented by fluid restriction and vasopressin administration. The immersion response is driven by suppression of antidiuretic hormone release, also known as the Gauer-Henry response. The urinary sodium excretion correlates with distention of the heart, but is related to a decrease in tubular sodium reabsorption and not to an increase in sodium filtration. Natriuresis involves aldosterone suppression via decreased renin-angiotensin activity, increased release of atrial natriuretic factor(s), release of renal prostaglandins, and decreased renal sympathetic activity.
The distension of the heart in immersion enhances ventricular diastolic filling (preload), which increases the cardiac output almost entirely due to an increase in stroke volume, which may double. The elevated cardiac output is sustained, but is not accompanied by an increase in oxygen consumption.
The immersion response has important implications for the physiological events related to breath-hold diving.2 While breath-holding, the inflation of the lungs provides a reservoir for the continued exchange of O2 for CO2. A breath-hold in air decreases the mean alveolar partial pressure of O2 (PO2) as a linear function of the decrease in mixed venous PO2. As alveolar PO2 falls, the O2 consumption remains constant until the O2 delivery reaches a threshold beyond which anaerobic metabolism increases. CO2 enters the lungs in proportion to pulmonary blood flow and the CO2 diffusion (PCO2) gradient between the mixed venous and alveolar partial pressures. Initially, the CO2 transfer rate is high, but falls as the alveolar PCO2 approaches the mixed venous PCO2. Continuing CO2 production increases the mixed venous PCO2, which then allows the alveolar PCO2 to increase further. The rising PCO2 causes breathing to resume at the so-called break point. The time to the break point can be extended by maneuvers that lower the PCO2 or raise the PO2 such as hyperventilation or O2 breathing. Hyperventilation does not appreciably increase the body’s O2 stores because the increase in alveolar PO2 resulting from a decrease in alveolar PCO2 only increases blood O2 content slightly. Thus, hyperventilation extends breath-hold time, but profound hypoxia may develop before the CO2 reaches the break point.
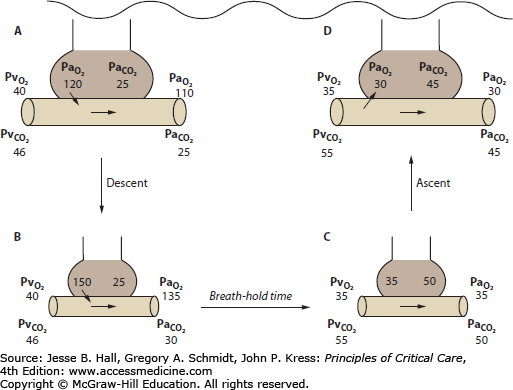
The physiology of breath-holding is altered by underwater descent because the thoracic compression decreases the lung volume, which increases the partial pressures of O2, CO2, and N2 in the lungs (Fig. 132-1). The alveolar O2 and CO2 concentrations decrease faster than the inert gas (N2) because those gases are transferred more quickly to the pulmonary capillary blood as O2 is consumed and since CO2 is more soluble than nitrogen.
FIGURE 132-1
Mechanism of shallow water blackout. All values are expressed in millimeters of mercury. A. The breath-hold diver hyperventilates before the dive to bring down the alveolar partial pressure of CO2 (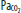 ). B. At depth, arterial CO2 (
). B. At depth, arterial CO2 (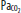 ) and O2 (
) and O2 (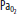 ) increase as the alveoli are compressed by hydrostatic pressure. C. At the end of the breath-hold dive, CO2 increases toward the break point, and the diver begins to ascend. Note the decreased
) increase as the alveoli are compressed by hydrostatic pressure. C. At the end of the breath-hold dive, CO2 increases toward the break point, and the diver begins to ascend. Note the decreased 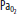 and
and 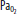 at this point. D. When approaching the surface,
at this point. D. When approaching the surface, 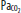 and
and 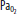 decrease as the alveoli reexpand; O2 leaves the pulmonary capillaries and enters the alveoli. This may result in profound hypoxemia and produce unconsciousness.
decrease as the alveoli reexpand; O2 leaves the pulmonary capillaries and enters the alveoli. This may result in profound hypoxemia and produce unconsciousness.
Compared with a simple breath-hold in air, the alveolar PO2 initially increases during a breath-hold dive due to the increase in pressure on the thorax. The transfer of CO2 during the early descent is opposite normal, and CO2 moves from alveoli to pulmonary capillary blood. During ascent, the lung re-expands, and as the pressure decreases, so do the alveolar PO2 and PCO2. Near the surface, the alveolar PO2 may approach the mixed venous PO2, and during ascent from particularly strenuous dives, the expansion of hypoxic alveoli may result in reverse O2 transfer from mixed venous blood to alveoli (see Fig. 132-1). Carbon dioxide in the blood during the dive also leaves during ascent as alveolar PCO2 decreases. Carbon dioxide elimination continues after the dive as does the elimination of the small amount of N2 that entered the blood during the dive.
Hyperventilation before a breath-hold dive is a dangerous way to extend the duration of dive. Because the alveolar PO2 initially increases from thoracic compression, the primary signal to return to the surface is the PCO2. If the diver hyperventilates before the breath-hold, the arterial PCO2 begins at a lower level, which extends the time to the break point. During the longer dive, the alveolar O2 concentration decreases to lower levels, and as the diver approaches the surface, life-threatening hypoxia and loss of consciousness may occur. Traditionally, this is called shallow water blackout, although free divers may refer to it as deep water blackout to distinguish it from unconsciousness after breath-hold swimming events performed entirely in shallow water, such as in pools.3 Both types of events are responsible for many episodes of drowning.
In breath-hold diving, the physiology is modified by a diving response induced by apnea and facial immersion, particularly if the water is cold. This diving response, manifested by the triad of apnea, bradycardia, and redistribution of organ blood flow is most pronounced in young children. It has been interpreted as an O2-conserving response that redistributes blood flow from organs resistant to hypoxia to organs with continuous O2 needs such as heart and brain. This diving response may convey a small advantage to trained human apnea divers, and it probably does contribute to the survival of children after submersion in cold water.
DIVING WITH COMPRESSED GASES
The practical utility of underwater breath-hold diving is limited by time and depth, although today there are still working free divers, such as the Japanese Ama, and competitive, “no-limit” apnea divers have achieved depths exceeding 200 m. The use of pressurized underwater breathing apparatus provides the diver with a continuous supply of breathing gas at almost any depth. In shallow water, for instance, 0 to 135 fsw, diving is usually carried out with compressed air because it is inexpensive and readily available, even at remote locations. Air divers are usually free swimming, that is, they use a self-contained underwater breathing apparatus (SCUBA). Special gas mixtures, such as 32% nitrogen-oxygen (nitrox), used to extend the bottom time and/or provide an extra safety margin, are increasingly being used by recreational divers.
The recommended maximum safe depth for Scuba divers is 135 fsw and approximately 200 fsw for divers tethered to a safety line.4 Safety concerns are brought about by three factors: nitrogen narcosis, safe decompression, and oxygen toxicity. The problems of importance to the intensive care specialist are related to decompression illness and pulmonary overpressurization as discussed below. The other problems are covered in standard textbooks on diving medicine.
To dive beyond the practical range of compressed air, special gas mixtures, such as helium-oxygen (heliox) or oxygen-enriched air (nitrox) must be supplied. In heliox operations, inspired oxygen pressure is usually maintained at a constant 0.4 to 1.0 ATA, and the helium may be recycled by gas reconditioning equipment. Surface-supplied heliox diving is expensive but effective and relatively safe to depths of 400 fsw. Most dives deeper than 400 fsw require saturation of the diver with inert gas at the approximate working depth of the dive. Saturation divers can live and work for weeks at pressure, for instance in a bell and chamber system, and then undergo a single slow decompression to the surface.
The principle pathophysiologic problems of compressed gas diving occur during ascent due to the uncontrolled emergence of inert gas from tissues. During an ascent from diving or to altitude the extra inert gas in the body at the higher pressure is eliminated as the pressure decreases by the process of decompression.5 The rates of uptake or elimination of inert gases from the body after a pressure change are determined primarily by the solubility of the gas in blood and tissue, the blood flow, and the volume of tissue. In most tissues, inert gas exchange follows an exponential function with respect to time. Tissues that behave this way are perfusion limited, and the characteristics of their inert gas exchange are defined by a half-time. Because the body tissues receive different amounts of blood flow and nitrogen is more soluble in fat, half-times for various tissues vary considerably. The principle of multiple tissues was used to calculate the first safe decompression tables, with the assumption that gas bubbles and DCS would occur only if the tissues were supersaturated to allow a nitrogen partial pressure of about twice the absolute pressure. Modern decompression tables are still based on such parallel exponential models, but with lower degrees of supersaturation. The most important variable affecting inert gas uptake and elimination is blood flow or tissue perfusion, but diffusion may limit tissue gas exchange under some conditions.5 Diffusion may become important when two adjacent tissues have very different rates of perfusion. In such conditions, the more highly perfused tissue eliminates inert gas more quickly, allowing inert gas to diffuse into it from the slower tissue. Thus, a faster tissue may remain supersaturated for longer than expected. Diffusion is also important during decompression once gas bubbles have formed in a tissue. Bubbles contain large amounts of N2 gas that can be removed by perfusion only after the N2 diffuses back into the tissue. The rate at which N2 diffuses away from a gas bubble is determined by the bubble surface area, the intrabubble pressure, and the difference in partial pressure between bubble and tissue.
Bubbles tend to form in specific nucleation sites in the body during decompression. Microscopic gas nuclei can be stabilized at hydrophobic sites in the body, but grow into bubbles during decompression. The number of nucleation sites and their location and propensity to form macroscopic bubbles differ according to physiologic conditions. For example, exercise may increase the number of bubbles formed by tribonucleation, a mechanism by which large negative pressures can generate bubbles by traction between surfaces lubricated by a liquid, such as joints.
Decompression illness encompasses both decompression sickness (DCS) and arterial gas embolism (AGE) and these conditions, particularly after an uncontrolled ascent by an inexperienced diver, may coexist. As a rule, AGE has more serious implications, and it is a medical emergency. Arterial gas blocking cerebral or coronary vessels and causing ischemia must be eliminated promptly for the best outcome.
DCS is attributable to the growth of bubbles in body tissues that produce one or more clinical manifestations. The most common presentation is pain-only or type I DCS, also known as bends. In type I DCS, the primary sites of bubble growth are the joint spaces, tendon sheaths, and periarticular tissues, including peripheral nerves. Type II or serious DCS is less common and usually involves the central nervous system, including the brain and spinal cord. Altitude DCS is similar, although symptoms appear most often during the exposure. Altitude DCS tends to be pain-only because the subject has often breathed an O2-enriched gas or has undergone O2 prebreathing. Although uncommon, cases involving the CNS do occur.
Most serious cases of DCS are due to omitted decompression and/or other risk factors such as exercise, cold, coexisting dehydration, or preexisting medical conditions or injuries that compromise regional blood flow. Other serious forms of DCS involving the audiovestibular system (staggers) and pulmonary system (chokes), although relatively rare, also occur. The most common clinical manifestations of DCS are presented in Table 132-2. The variable manifestations can make the clinical diagnosis difficult to establish, and there are no diagnostic laboratory studies.
Clinical Manifestations of DCS
|