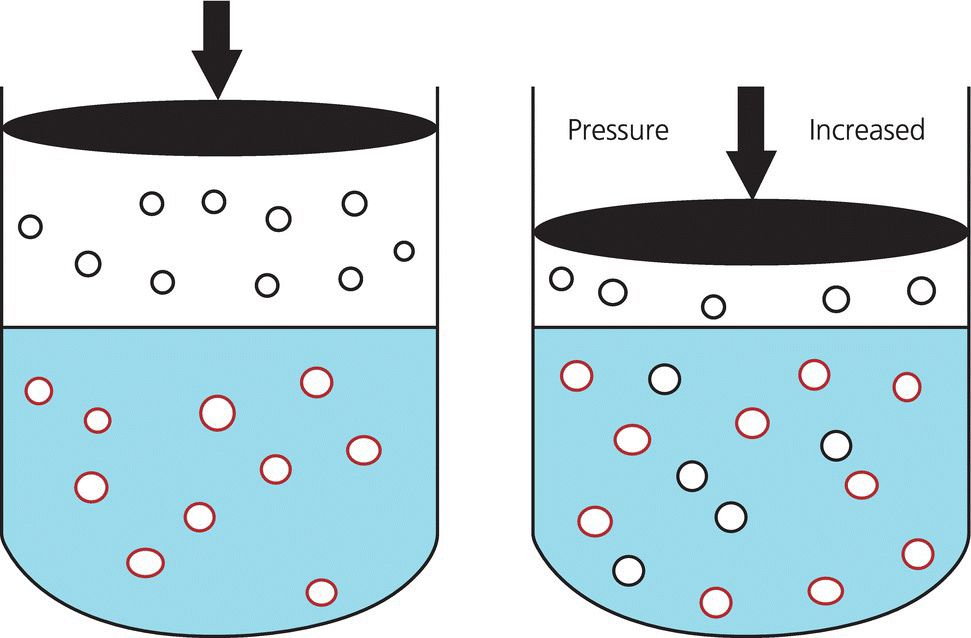
Chapter 52 Anthony J. Frank Jr Water comprises 70% of the surface of our planet. It is only natural that given this large percentage of our home, human beings would be drawn to explore this environment. It is currently estimated that there are 1.2 million active scuba divers worldwide [1] and that approximately 200,000 new divers are certified every year [2]. Although diving is considered to be a relatively safe sport, operating in an environment with unique hazards where life-supportive breathing gases must be carried leaves little margin for error. Comparing the total hours involved, diving is estimated to be 96 times more dangerous than operating a motor vehicle [3]. Emergency medical services physicians and medical directors of EMS systems need not be certified scuba divers, but will benefit from developing a fundamental knowledge of dive-related physiology and hazards. There are four main categories of diving injury: injuries on the surface, injuries of descent, injuries at depth, and injuries of ascent. This chapter deals with injuries below the surface. Due to the high density of water, small changes in depth cause significant changes in the pressure exerted on an object. At the surface, a body is subjected to the weight of the earth’s atmosphere, which is equal to 1 atmosphere absolute (ATA). During descent, for every 33 feet of seawater (fsw) or 34 feet of freshwater (ffw) traveled below the surface, pressure increases by 1 atmosphere (atm). Typical units of measure for pressure include: 33 fsw = 34 ffw = 1 atm = 760 mmHg = 760 torr = 14.7 psi. The majority of recreational diving occurs between 33 and 120 fsw (2 to <5 ATA). To appreciate the physiology of diving injury, EMS physicians must be familiar with Boyle’s law, Dalton’s law, and Henry’s law. Governing the physiology of barotrauma and recompression therapy, Boyle’s law states that given a constant temperature, the volume and pressure of an ideal gas are inversely related. It also deals with conditions related to changes in pressure in hollow, air-filled organs and structures in the body. As an example, during descent the pressure is doubled; on a descent from the surface (1 ATA) to a depth of 33 fsw (2 ATA), the volume of a gas is halved (Figure 52.1). The law is typically stated as: Figure 52.1 Boyle’s law. As a diver is descending in the water column, the volume of air in gas-filled organs will decrease. If the volume of air in the lungs at the surface is V then at 33 fsw the volume will be 1/2 V, at 66 fsw 1/3 V, etc. If using compressed air, as in scuba diving, when a breath is taken at 66 fsw, lung volume returns to V. If ascent occurs at this point without exhalation, as in an unconscious diver, the lung volume will expand to 1.5 V at 33 fsw and 3 V at the surface, with potential for barotrauma. Liquids and liquid-filled organs are non-compressible. The body tissues are composed primarily of water and thus there is no change in volume with pressure increases and decreases. Dalton’s law explains the physiology of conditions such as oxygen toxicity and nitrogen narcosis. The law states that total pressure exerted by a mixture of gases is the sum of the partial pressures of the gases in the mix. Thus for fresh air: As total pressure is increased, the partial pressures of each gas in the mixture will increase proportionally. Fresh air is composed of 79% nitrogen and 21% oxygen. These ratios remain constant as pressure is increased at depth. The partial pressure of nitrogen in air at sea level is approximately 600 mmHg or 0.79 ATA (0.79 × 760 mmHg), and of oxygen is 160 mmHg or 0.21 ATA (0.21 × 760 mmHg). At a depth of 66 fsw, the partial pressure of each would be 3 × 600 = 1800 mmHg (2.37 ATA) for nitrogen and 3 × 160 = 480 mmHg (0.63 ATA) for oxygen. Henry’s law is the foundation for decompression sickness (”the bends”). The law states that at equilibrium, the concentration of a gas dissolved in a liquid is directly proportional to the partial pressure of the gas above the liquid (Figure 52.2). This is stated as: Figure 52.2 Henry’s law. where P is the partial pressure of the gas above the liquid, k is a constant, and C is the concentration of the gas in the liquid. The common example is opening a carbonated beverage container. As the pressure is reduced by opening the can, the CO2 dissolved in solution escapes, forming bubbles as the gas equalizes with the atmospheric partial pressure of the gas. Barotrauma is an injury that occurs due to changes in pressure of an air-filled structure during descent or ascent. Barotrauma is the most common medical problem associated with diving and can involve almost any structure that can have entrapment of gases. Barotrauma causes injury by a change in volume of free gas in an air-filled organ resulting in a pressure disequilibrium. Both increasing and decreasing pressure can cause mechanical injury to body structures. Pain is typically the initial complaint. Middle ear barotrauma, also known as barotitis media or “ear squeeze,” is the most common complaint and medical problem of scuba divers. It is experienced by 30–40% or novice scuba divers and 10% of experienced divers [4]. As a diver descends in the water column, water pressure against the tympanic membrane (TM) increases. A diver will employ various methods to force air into the middle ear through the eustachian tube to equalize this pressure across the TM. If the diver is unsuccessful at “clearing” his ears, continued attempts may be futile due to the collapsible nature of the medial third of the eustachian tube. Ascent and reattempts at clearing are the only option for resolution. Further descent may cause TM rupture and result in cold water caloric stimulation and vertigo which may precipitate panic, disorientation, rapid ascent with other associated types of barotraumas, or drowning. Treatment of middle ear barotrauma is usually with decongestants and analgesics and it will typically resolve over 3–7 days [5]. Refraining from diving with a cold or other symptoms that may cause difficulty with pressure equalization and early recognition of symptoms are common prevention strategies. Inner ear barotrauma is much less common than middle ear barotrauma, but has a higher morbidity. Damage to the cochleovestibular apparatus is the result of a large negative pressure gradient in the middle ear that occurs due to a forceful Valsalva maneuver against an occluded eustachian tube. As a result of the increased pressure during the attempted Valsalva, the pressure differential between the cerebrospinal fluid through the vestibular and cochlear structures and the middle ear may result in several injuries, including round or oval window rupture, middle ear hemorrhage, Reissner’s membrane tear, fistualization of the windows, or a combination of these. A triad of findings are associated with inner ear barotrauma: vertigo, unilateral roaring tinnitus, and hearing loss. In addition, a feeling of ocular fullness, nystagmus, disorientation, ataxia, and nausea and vomiting may be seen. Immediate concerns relate to the occurrence of panic in the underwater environment, uncontrolled ascent, or drowning. Treatment of inner ear barotrauma includes elevating the head of the bed to 30°, bed rest, avoidance of strenuous physical activity, and symptomatic treatment. Early otolaryngology consultation should be obtained for further treatment recommendations as surgical repair options remain relatively controversial [6]. A less common condition than middle ear barotrauma, external squeeze may occur with a tight-fitting wetsuit hood creating a relative negative pressure in the external canal. The TM is pulled outward due to trapped air in the canal. Cerumen, earplugs, and structural abnormalities may also contribute to this condition. The sinus cavities are susceptible to pressure-volume changes according to Boyle’s law. The ethmoid, maxillary, and frontal sinuses require patent nasal passages for equalization of pressure. Any mechanical abnormalities such as deviated septum, polyps, or physical conditions such as bacterial and viral infection, or upper respiratory infections may predispose a diver to sinus barotrauma and resultant barosinusitis. The frontal sinus is the most commonly affected sinus cavity due to the long connection to the nasal passage [4]. Common signs and symptoms in barosinusitis include facial pain or fullness during descent or ascent, numbness to the front of the face, upper tooth pain, and epistaxis. Systemic decongestants and topical nasal vasoconstrictors are the mainstay of treatment. Some authors advocate a short corticosteroid burst to hasten recovery and return to diving [5].
Diving injury
Historical perspective
Introduction
The gas laws
Boyle’s law
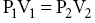
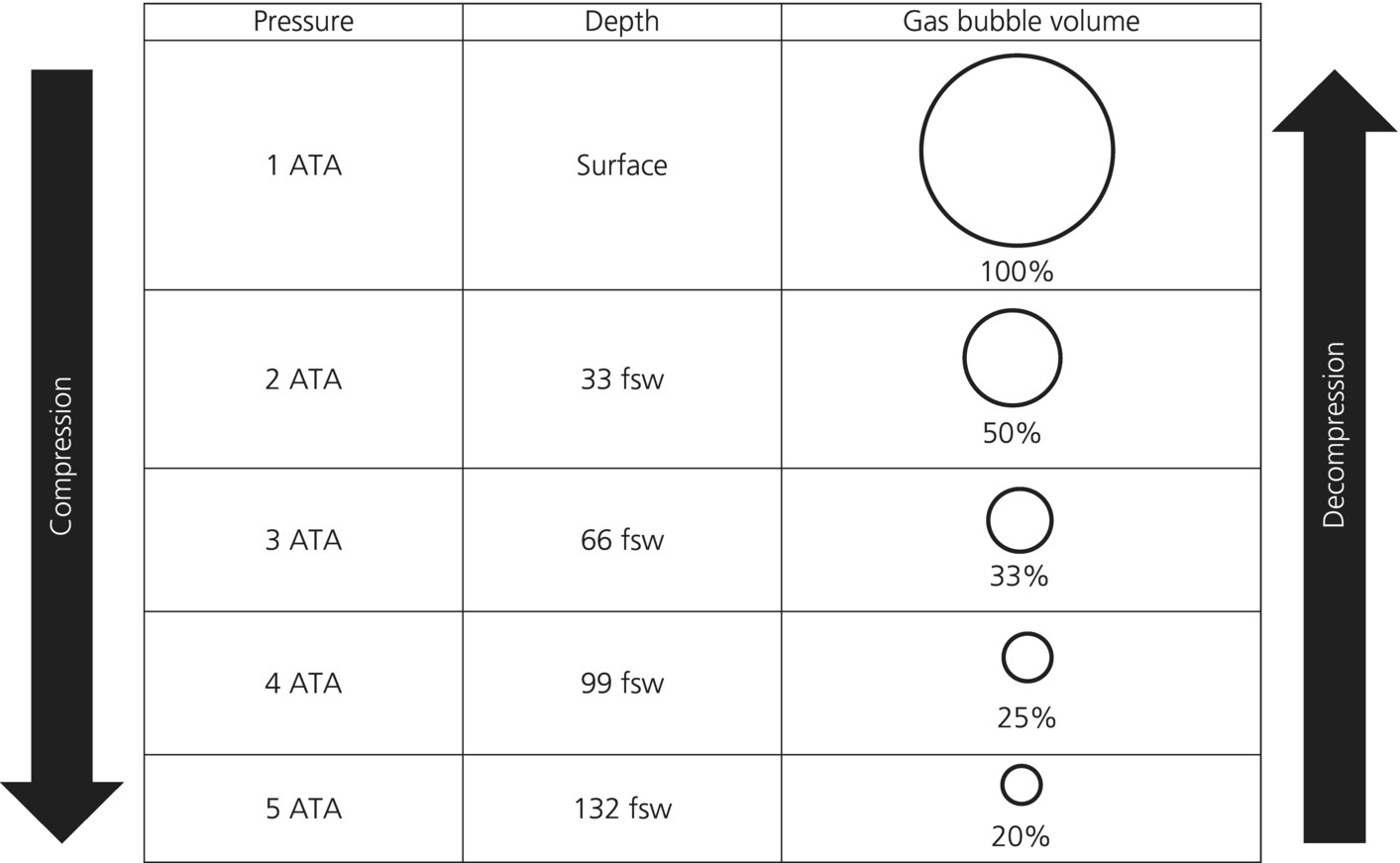
Dalton’s law
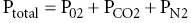
Henry’s law
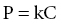
Injury of descent: barotrauma of descent
Middle ear barotrauma
Inner ear barotrauma
External ear barotrauma
Sinus barotrauma
Mask squeeze
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





