Disorders of Water, Sodium, and Potassium Homeostasis
James Schneider
Andrea Kelly
KEY POINTS
 Disorders of water, Na+, and K+ occur commonly in the pediatric intensive care unit, and these disturbances of fluid and electrolyte homeostasis are generally associated with underlying comorbidities, such as renal dysfunction, diabetic ketoacidosis, diabetes insipidus (DI), chemotherapy, brain tumors, and brain surgery.
Disorders of water, Na+, and K+ occur commonly in the pediatric intensive care unit, and these disturbances of fluid and electrolyte homeostasis are generally associated with underlying comorbidities, such as renal dysfunction, diabetic ketoacidosis, diabetes insipidus (DI), chemotherapy, brain tumors, and brain surgery. Evaluation, assessment, and management of fluid and electrolyte disorders require an understanding of the basic factors that regulate homeostasis as well as the differential diagnosis of the underlying conditions that predispose to altering water, Na+, and K+ homeostasis.
Evaluation, assessment, and management of fluid and electrolyte disorders require an understanding of the basic factors that regulate homeostasis as well as the differential diagnosis of the underlying conditions that predispose to altering water, Na+, and K+ homeostasis. Therapeutic management requires both managing the underlying condition appropriately and being aware that inappropriate therapeutic measures can be an iatrogenic cause of disturbance of fluid and electrolyte homeostasis.
Therapeutic management requires both managing the underlying condition appropriately and being aware that inappropriate therapeutic measures can be an iatrogenic cause of disturbance of fluid and electrolyte homeostasis. In the clinical acute care setting, careful historic information and physical examination are the cornerstone of therapy; however, it must be stressed that physical assessment of the degree of dehydration or hydration is, at best, only an initial estimation; that vital signs, clinical improvement, and biochemical parameters must be monitored closely; and that changes in fluid therapy must be allowed for as necessary.
In the clinical acute care setting, careful historic information and physical examination are the cornerstone of therapy; however, it must be stressed that physical assessment of the degree of dehydration or hydration is, at best, only an initial estimation; that vital signs, clinical improvement, and biochemical parameters must be monitored closely; and that changes in fluid therapy must be allowed for as necessary. Often, the most complicated disorders of water, Na+, and K+ to manage are those in which the basic underlying conditions are changing, such as in syndrome of inappropriate antidiuretic hormone, postoperative craniopharyngioma patients, or patients with known central DI subsequently developing a salt-wasting condition. Frequent laboratory monitoring is exceptionally important in management of these children.
Often, the most complicated disorders of water, Na+, and K+ to manage are those in which the basic underlying conditions are changing, such as in syndrome of inappropriate antidiuretic hormone, postoperative craniopharyngioma patients, or patients with known central DI subsequently developing a salt-wasting condition. Frequent laboratory monitoring is exceptionally important in management of these children. Clear potential for morbidity and mortality exists with any disturbance of water, Na+, and K+ homeostasis, but perhaps the most common abnormality that results in injury and death is hyponatremia. It must be stressed, however, that prolonged and untreated hypernatremia is also associated with significant injury and death.
Clear potential for morbidity and mortality exists with any disturbance of water, Na+, and K+ homeostasis, but perhaps the most common abnormality that results in injury and death is hyponatremia. It must be stressed, however, that prolonged and untreated hypernatremia is also associated with significant injury and death. Recent studies stressed the need to avoid the use of hypotonic fluids during resuscitation, as they are a major cause of iatrogenic hyponatremia. Further, the continued use of hypotonic fluids as maintenance therapy is in question, as critically ill children often have the inability to optimally dilute urine, increasing the risk for the development of hyponatremia.
Recent studies stressed the need to avoid the use of hypotonic fluids during resuscitation, as they are a major cause of iatrogenic hyponatremia. Further, the continued use of hypotonic fluids as maintenance therapy is in question, as critically ill children often have the inability to optimally dilute urine, increasing the risk for the development of hyponatremia.Children in the pediatric intensive care unit (PICU) are frequently admitted with or, even more commonly, develop disorders of water, sodium (Na+), or potassium (K+). Water or electrolyte abnormalities may be manifestations of the principle disease process but more often arise from either secondary organ injury or from interventions, such as medications, fluid management, or positive pressure ventilation. The intensivist plays a vital role in recognizing these risks and monitoring for development of these disorders. It is becoming clearer that fluid or electrolyte imbalances have an important influence on the outcome of critically ill children, and, therefore, a thorough understanding of the normal physiology and pathophysiology that leads to these imbalances is fundamental to providing optimal care. Discussions in this chapter focus on the evaluation and management of water, Na+, and K+ disorders as they pertain to the critically ill child and will not attempt to be inclusive of all such disorders in less critical settings. Although mentioned in various sections, molecular mechanisms, genetic alterations, animal models, and similar information will not be discussed in detail. Further, sophisticated laboratory studies not generally immediately available to the intensivist will be discussed to only a limited degree. Specifically, discussions will focus on paradigms regarding the clinical evaluation and management of fluid disorders, as appropriate correction of fluid disturbances in the critically ill child is often paramount for resuscitation and survival.
To provide the foundation for an understanding of the pathophysiologic disturbances, this chapter will include a review of normal physiology and the mechanisms that regulate water, Na+, and K+ balance. The disorders of water, Na+, and K+ will be discussed as to etiology and most common presentations in the critical care setting. As to the differential diagnosis of the various conditions, the discussions will focus upon the most probable causes that result in admission to a PICU. Fluid management will be discussed, including the differences in initial therapies involving resuscitation, as well as maintenance of hydration and electrolyte control. A review of outcomes related to these electrolyte or fluid disturbances will also be presented as a basis for discussion of potential sources of error.
NORMAL PHYSIOLOGY AND PATHOPHYSIOLOGY
Strict regulation of total body water, Na+, and K+ occurs  through multiple, often redundant, pathways. Regulation of Na+ is critical for maintaining extracellular fluid (ECF)
through multiple, often redundant, pathways. Regulation of Na+ is critical for maintaining extracellular fluid (ECF)
volume, while regulation of K+ is vital for maintaining cellular electrophysiology. Superimposed upon regulation of these electrolytes is water metabolism, which is primarily influenced by changes in serum osmolality and, to a lesser extent, volume status. The kidney is the primary site for regulation of Na+, K+, and water, a result of the interplay between multiple hormonal pathways (Fig. 107.1).
 through multiple, often redundant, pathways. Regulation of Na+ is critical for maintaining extracellular fluid (ECF)
through multiple, often redundant, pathways. Regulation of Na+ is critical for maintaining extracellular fluid (ECF) volume, while regulation of K+ is vital for maintaining cellular electrophysiology. Superimposed upon regulation of these electrolytes is water metabolism, which is primarily influenced by changes in serum osmolality and, to a lesser extent, volume status. The kidney is the primary site for regulation of Na+, K+, and water, a result of the interplay between multiple hormonal pathways (Fig. 107.1).
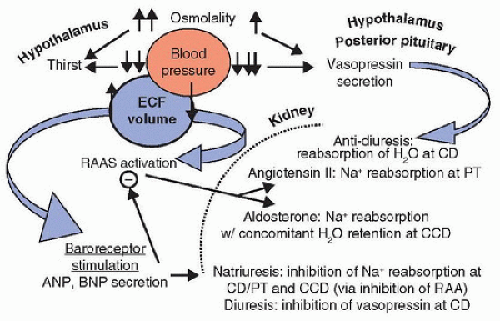 FIGURE 107.1. Regulation of extracellular fluid osmolality and volume. Vasopressin secretion is primarily responsible for preserving plasma osmolality. Secretion of vasopressin by the hypothalamus occurs with as little as a 1% increase in plasma osmolality. Much larger increases in plasma osmolality are required to trigger thirst, the center for which is also located in the hypothalamus. This offsetting likely occurs to avoid simultaneously activating thirst and vasopressin secretion at the lower end of normal plasma osmolality, which would result in overcorrection. Significant decreases in blood pressure/effective extracellular fluid (ECF) volume, communicated to the hypothalamus via cardiovascular baroreceptors, are required to trigger vasopressin secretion. Vasopressin recruits aquaporin-2 water channels in the renal collecting ducts (CD) to promote reabsorption of water and concentration of urine. ECF volume is primarily maintained through Na+ homeostasis. Decreases in blood pressure/effective ECF volume also activate the renin-angiotensin-aldosterone system (RAAS). Aldosterone promotes reabsorption of Na+ and water at the renal cortical-collecting duct (CCD), and angiotensin II stimulates Na+ reabsorption at the proximal tubules (PT). Hypertension/fluid overload activates cardiovascular baroreceptors, leading to A-type natriuretic peptide (ANP) and brain natriuretic peptide (BNP) release. These peptides promote Na+ and water excretion at the level of the kidney. |
Renal Handling of Water and Solutes—an Overview
A number of renal factors influence the dilution and concentration of urine. First, the glomerular filtration rate (the amount of renal blood flow that enters the nephron) dictates the maximum amount of fluid that can be delivered to the tubules. The glomerular filtration rate is heavily influenced by renal blood flow through the afferent and efferent arterioles. Vasoconstriction of afferent arterioles decreases glomerular blood flow, thereby decreasing glomerular pressure and filtration, while vasoconstriction of efferent arterioles, to an extent, increases glomerular pressure and filtration. A feedback mechanism, mediated by the macula densa, controls the vasoconstriction and vasodilation of the afferent and efferent arterioles to autoregulate the glomerular filtration rate. When the macula densa “senses” a low glomerular filtration rate, vasodilatation of afferent arterioles (and vasoconstriction of efferent arterioles mediated by angiotensin II) increases glomerular perfusion, thereby increasing the glomerular filtration rate to affect urine output. Conversely, with increased glomerular filtration, the macula densa again provides negative feedback to the afferent and efferent arterioles: vasoconstriction of afferent arterioles (and vasodilatation of efferent arterioles) occurs, decreasing glomerular filtration, slowing fluid transport through the nephron, and allowing increased filtrate reabsorption and, ultimately, decreased urine output. Afferent and efferent arterioles are regulated by the sympathetic nervous system and angiotensin II, as described later.
Following filtration at the level of the glomerulus, the fluid is delivered to the tubules, where solute absorption varies depending upon the segment. The proximal tubule accounts for 65% of filtrate reabsorption, including that of Na+, K+, and water. The descending thin limb of the loop of Henle is permeable to water, urea, and other solutes, while the ascending thin limb is relatively impermeable to water. The thick ascending limb (TAL) and the initial segment of the distal tubule avidly absorb Na+ and other solutes but are impermeable to water regardless of the status of vasopressin and, hence, are referred to as the diluting segment of the kidney. Here, tubular fluid osmolality falls below that of the glomerular filtrate. The late distal tubule and cortical-collecting duct mediate the Na+-retaining and K+-wasting effects of aldosterone. In addition, like the collecting duct, they are permeable to water only in the presence of vasopressin.
Na+ absorption occurs through an active process and facilitates the absorption of many other solutes. Na/K-ATPase, expressed on the basolateral membrane of tubular epithelium, pumps Na+ into the renal interstitium and ultimately to the peritubular capillaries (Fig. 107.2). Activity of this enzyme generates an Na+ concentration gradient and an electrochemical gradient between the tubule lumen and the tubular cell. These gradients facilitate Na+ transport from tubular fluid into the tubular epithelium, further supplying Na+ for the Na/K-ATPase pump. In addition, these gradients drive water reabsorption. Because the cellular concentration of K+ is already high relative to interstitial fluid, and because the tubular epithelial basolateral membrane is readily permeable to K+, K+ driven into the cell by Na/K-ATPase diffuses easily into the interstitium for reabsorption from the filtrate.
From this discussion, it can be concluded that water, Na+, and K+ homeostases are not completely independent.
A disorder that primarily affects one of these important body constituents can have a significant impact on another.
A disorder that primarily affects one of these important body constituents can have a significant impact on another.
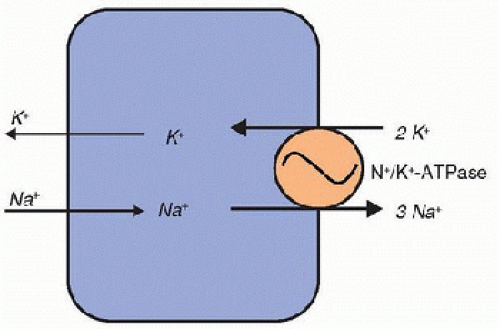 FIGURE 107.2. Na/K-ATPase activity. The Na/K-ATPase is ubiquitously expressed on the plasma cell membrane. Via an ATP-consuming process, this enzyme exchanges three intracellular Na+ cations for two extracellular K+ cations to maintain the intracellular-to-extracellular gradients for these two cations. Na+ can pass down this concentration gradient from the extracellular fluid (ECF) to the intracellular fluid (ICF), whereas K+ diffuses down its concentration gradient from the ICF to the ECF. The location of these pumps varies depending upon the cell type; they may be distributed evenly along the cell membrane or localized to the basolateral membranes as in renal epithelial cells. |
Water Homeostasis
The human body is composed of 42%-75% water, with the exact content dependent upon age, gender, and amount of body fat. Approximately two-thirds of the water is located intracellularly. The remaining estimated one-third is located extracellularly and is divided between the interstitium (threefourths) and plasma (one-fourth) (Fig. 107.3). The solute content of these fluid compartments differs significantly, with K+ being the primary constituent of intracellular fluid (ICF) and Na+ being the major electrolyte in ECF. Na/K-ATPase, a plasma membrane enzyme expressed on most cells, is responsible for maintaining this discordant distribution of Na+ and K+




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




