Key Clinical Questions
What is the normal size of the aorta, common femoral artery, iliac artery, and popliteal artery?
What do the American College of Cardiology and American Heart Association Guidelines recommend for abdominal aortic aneurysm screening?
What is the preferred classification system for aortic dissection?
When should large vessel vasculitis be suspected?
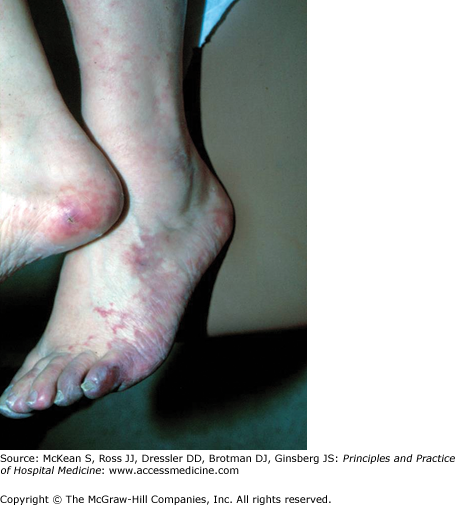
What are the causes of blue toe syndrome and what is the diagnostic modality of choice?
Introduction
Acute and chronic aortic syndromes have been the subject of intense investigation by physicians for centuries. These examinations have yielded a wealth of knowledge about the pathology, pathobiology, and pathophysiology of diseases of the aorta. More recent technological advancements have significantly improved our understanding of aortic syndromes at the molecular level, and have also helped in their accurate and timely initial diagnosis and long-term management. In addition, large clinical databases have elucidated the epidemiology and furthered the understanding of the natural history of many different aortic syndromes. However, despite the current armamentarium of knowledge and technology at the physician’s disposal, the accurate diagnosis of acute aortic syndromes is not always apparent, sometimes with disastrous consequences. This chapter will focus on the presentation, diagnosis, and management of the various forms of acute and chronic aortic diseases, including aortic dissection and its variants, abdominal and thoracic aneurysms, multiple atheromatous embolization, giant cell and Takayasu arteritis, and blue toe syndrome (Figures 262-1 and 262-2).
Acute Aortic Disorders: Aortic Dissection and Its Variants
Acute aortic disorders include acute aortic dissection and its variants of intramural hematoma and penetrating atherosclerotic ulcer. All of these represent disruptions of one or more of the three layers of the aorta: the innermost intimal layer; the middle layer, or media; and the outermost adventitial layer. The intima is a very thin, endothelial-lined layer in its luminal portion, and it is prone to traumatic insults. The media is thick walled and contains multiple layers of elastic laminae (elastin, fibrin, collagen) and smooth muscle cells. The adventitia is primarily composed of collagen that helps to anchor the aorta to it surroundings, and also contains the vasa vasorum that functions to deliver blood to the outer aortic wall and to the media. The common pathway in all three acute aortic syndromes is a preceding weakening of the layers of the aorta that may lead to a tear in the intima, as in the case of dissection; rupture of the vasa vasorum, which leads to aortic infarction but with intact intima, as in the case of intramural hematoma; and atherosclerotic ulcer, which penetrates into the adventitia with surrounding hematoma. The weakening of the aortic layers occurs via various mechanisms including atherosclerosis, inflammation, cystic medial necrosis (deposition of mucoid material), disruption of the elastic lamellae, fibrosis of collagen, and medionecrosis and apoptosis of vascular smooth muscle cells.
The incidence of aortic dissection is approximately 2.6–3.5 cases per 100,000 person-years, or approximately 2000 cases per year in the United States. In a review of the International Registry of Acute Aortic Dissections (IRAD), most affected individuals were male, with peak incidence in the sixth and seventh decades of life. Women who present with an acute dissection are usually older (mean age 67) than their male counterparts (mean age 63). In contrast, patients with intramural hematoma and atherosclerotic penetrating ulcers (which occur almost exclusively in the abdominal aorta) are even older (mean age 77), reflecting long-term atherosclerotic damage of the aortic wall. The mortality for aortic dissection depends on the location of the dissection, but overall mortality for untreated acute aortic dissection is approximately 1–2% per hour from onset, within the first 48 hours. The most common origin of aortic dissection occurs in the ascending aorta (65%), with subsequent 20% occurring in the descending aorta, 10% in the aortic arch, and the rest in the abdominal aorta.
The most common risk factor for both aortic dissection and intramural hematoma is chronic hypertension, with approximately 75% of patients presenting with acute dissection reporting a history of hypertension. Increased age, smoking, hyperlipidemia, blunt trauma, cocaine use, and atherosclerosis are also important risk factors Pregnancy is a significant, under-recognized risk factor for aortic dissection insofar as up to 50% of dissections that arise in women less than 40 years old occur in the third trimester of pregnancy or in the early postpartum period. The mechanism for dissection is believed to be linked to pregnancy-related hemodynamic changes, pregnancy-induced hypertension, changes in the hormonal milieu with the release of relaxin, and underlying hypertension or connective tissue disorders. Young patients (< 40 years of age) who present with dissection are more likely to have genetically inherited conditions such as Marfan syndrome (defect in the fibrillin gene resulting in breakdown of elastic components of aortic wall), Ehlers-Danlos syndrome (defect in type III collagen in the aortic wall), Loeys-Dietz syndrome, aortic coarctation, or bicuspid aortic valve. A previous history of cardiac surgery occurred in 18% of patients presenting with dissection in the IRAD registry. In addition, disorders that are associated with vascular inflammation that leads to medial necrosis, such as giant cell arteritis, Takayasu arteritis, syphilis and Behcet, can also predispose to aortic dissection. Iatrogenic causes of aortic dissection include instrumentation of the aorta due to cardiac catheterization, intraaortic balloon pump placement, and cardiac surgery.
Aortic dissection is characterized by a tear in the intima and the development of an intimal flap, thus creating a true and a false lumen. There are two common classification schemes of aortic dissection: DeBakey and Stanford. Within the DeBakey scheme, type I dissection describes a dissection that involves the full span of the aorta, originating in the aortic root, and propagating to the abdominal aorta. Type II dissection originates in and is confined to the ascending aorta through the level of the origin of the innominate artery. The management and treatment of Types I and II are identical, as they both involve the ascending aorta. Type III dissection originates in the descending aorta, distal to the left subclavian artery, and extends either distally down the aorta or retrograde to the aortic arch. Within the Stanford scheme, type A dissection is any dissection that involves the ascending aorta, regardless of site of origin. Type B dissection is any dissection that does not involve the ascending aorta. The DeBakey types I and II are equivalent to Stanford type A, and the DeBakey type III is equivalent to the Stanford type B. Since the management of DeBakey types I and II are the same, the Stanford classification system has become the preferred nomenclature.
Aortic intramural hematoma is a variant of aortic dissection and presents in an identical manner clinically, but is characterized pathologically by the absence of a detectable intimal tear. The false lumen is usually caused by rupture of the vasa vasorum into the media of the aortic wall, thus causing aortic infarction. The second cause of intramural hematoma is a penetrating atherosclerotic ulcer, which will be described below. Aortic intramural hematoma is considered to be a precursor of aortic dissection, accounting for 5–20% of patients presenting with symptoms of aortic dissection. In addition, intramural hematomas can result in classic aortic dissection, with the aortic wall infarct that can cause a secondary intimal tear. Aortic intramural hematomas have a natural history that is similar to aortic dissections, although up to 10% have been noted to resorb spontaneously. Aortic intramural hematomas are classified according to the Stanford scheme of aortic dissections. Therefore, type A intramural hematomas occur in the ascending aorta. More commonly, intramural hematomas occur in the descending aorta and are classified as type B.
Penetrating atherosclerotic ulcers are atherosclerotic plaques that ulcerate into the media, leading to intramural hematomas and potential dissection. Atherosclerotic ulcers are almost exclusive to the abdominal aorta, and are associated with a higher rate of progressive disease (rupture, hematoma expansion, dissection) with medical treatment compared to aortic hematoma without ulcer. Separation of intramural hematoma, penetrating atherosclerotic ulcers, and classic aortic dissection on clinical grounds is not possible since all three entities present in the same fashion. Therefore the diagnosis is made on the basis of imaging techniques.
Patients with aortic dissection, intramural hematoma, and atherosclerotic penetrating ulcer present with similar symptoms and have a variety of clinical manifestations. According to the IRAD registry, the most common symptom is sudden onset of sharp chest and/or back pain, in contrast to the classic teaching of tearing or ripping pain. The pain is severe at its onset and stays constant, as opposed to the pain of myocardial infarction, which is less severe at onset and gradually intensifies. Chest pain is more commonly reported with Stanford type A dissections and intramural hematomas, whereas back or abdominal pain is more commonly associated with Stanford type B dissections and penetrating atherosclerotic ulcers. Less common presentations include heart failure, most commonly secondary to acute aortic regurgitation in Stanford type A dissections, and syncope, which occurs in up to 13% of proximal dissections. The etiology of syncope may be due to rupture into the pericardial space causing tamponade, activation of cerebral baroreceptors, cerebrovascular accident, paraplegia (due to involvement of intercostal arteries causing spinal cord infarct), or cardiac arrest. An important point for clinicians is that up to 30% of patients are misdiagnosed as having other potentially fatal conditions such as myocardial infarction, since Stanford type A lesions can dissect most commonly into the right aortic cusp and the right coronary artery, thus causing inferior ST elevations and a rise in cardiac biomarkers. Consequences of branch artery dissection include stroke, renal and mesenteric infarction, and limb ischemia.
Although hypertension is the most common predisposing factor for dissection and intramural hematoma, its absence does not exclude the diagnosis; at presentation, hypertension occurs in 36% of patients with type A and 70% of type B dissections. Hypotension is most commonly seen in proximal dissections with aortic root involvement, hemopericardium, and tamponade. Pulse deficits are helpful in diagnosis of acute aortic syndromes, but according to the IRAD registry, they occur in up to 30% of patients with type A and up to 21% of type B dissections. A diastolic murmur of aortic regurgitation, which reflects aortic root involvement with incomplete aortic valve coaptation, occurs in up to 44% of type A and 12% of type B dissections. Therefore, performance of a complete physical exam is essential in the evaluation of patients with chest pain.
An electrocardiogram, to help differentiate from acute myocardial infarction, and chest X-ray should be performed in all patients. A chest X-ray can demonstrate a widened mediastinum or displaced calcification in up to 85% of patients. Magnetic resonance imaging is the test with the highest sensitivity and specificity, but it is utilized in less than 5% of patients according to IRAD, as it has limited availability and takes longer to perform when compared with other modalities. The most common diagnostic tool is spiral computed tomography (CT), as it is fast and readily available in most centers in the United States. It has a sensitivity exceeding 95% and a specificity of 87–100%. In addition, CT can help with a “triple rule-out,” with evaluation of dissection, pulmonary embolus, and proximal coronary artery disease all at once. However, administration of iodonated contrast makes patient selection important when kidney disease is present. Transthoracic echocardiography is of limited use, as technical considerations such as mechanical ventilation, patient body habitus, and emphysema reduce its sensitivity to 59–85%, and specificity to 63–96%. Transesophageal echocardiography (TEE) has a sensitivity of 99% and a specificity of 89%, and has the advantage of being performed quickly. However, it does require conscious sedation, making patient selection important. Aortography is more technically challenging than other diagnostic modalities, and should be performed when diagnosis is not obtained with other tools or when evaluation of coronary anatomy directly impacts surgical considerations.
Along with admission to the intensive care unit, the major principles of initial management of both Type A and Type B lesions are decreasing the heart rate to < 60 beats per minute and decreasing the arterial blood pressure to between 100 and 120 mm Hg. This decreases the rate pressure product and thus reduces the impact of shear stress on the aortic vessel wall, which then decreases the extension of dissection and reduces the possibility of rupture. Initial therapy includes intravenous (IV) infusion of beta-blockers. Labetolol is the most frequently used beta-blocker, as it also has alpha-blocking properties that result in decrease in left ventricular contractility as well as systemic blood pressure. Esmolol, a beta-blocker with short half-life (0.13 hours) can be utilized in patients going to immediate surgery, or who have chronic obstructive pulmonary disease (COPD). In patients with absolute contraindications to beta-blocker therapy (eg, COPD with severe bronchospasm), IV calcium channel blockers such as verapamil or diltiazem are appropriate alternatives, but dihydropyridines alone should be avoided. If beta-blockers are not sufficient to reach target heart rate and blood pressure goals, IV vasodilators such as sodium nitroprusside are appropriate add-on therapy. However, these should not be used as monotherapy, as they can cause a reflex increase in heart rate thus increasing shear stress on the aortic wall. Pain control with IV morphine sulfate is an important part of initial management, and is frequently overlooked.
Patients with type A dissections should be taken emergently to surgery upon diagnosis since mortality increases 1–2% per hour upon initial symptom onset, and carries a mortality rate of 30% by 48 hours with medical management alone. The primary goal of surgery is to prevent the major complications of proximal dissections including myocardial infarction, aortic regurgitation, and cardiac tamponade. Some of the factors that contribute to higher in-hospital mortality include advanced age (> 70), hypotension, neurologic impairment, previous infarction, or valve surgery. Long-term treatment of these patients is similar to long-term treatment of patients with type B dissections, described in the next section.
Long-term treatment of most patients with type B lesions involves aggressive medical therapy. In a series of 384 patients from IRAD, 73% were managed medically, with a 10% in-hospital mortality. Aggressive blood pressure control (goal < 120/80 mm Hg) is achieved with oral agents such a beta-blockers or nondihydropyridine calcium channel blockers. If blood pressure is not at goal, vasodilators such as hydralazine or dihydropyridines may be effective add-on therapy. However, these should not be used as monotherapy as they also have the potential to cause a reflex increase in heart rate with subsequent increase in rate-pressure product. Surgical intervention is indicated for patients who have persistent or recurrent pain, uncontrolled hypertension, develop end-organ damage, dissection progression, or aortic expansion. Endovascular repair (percutaneous stent-grafts or aortic fenestration) has been utilized for treatment of unstable type B dissections, with the goal of closing the entry of blood into the false lumen, thereby causing its decompression and thrombosis.
Thirty percent of late deaths arise due to rupture of a secondary aneurysm that occurs within two years of initial treatment, or due to progression of dissection. Indeed, 20–30% of patients develop aneurysmal dilatation of type B dissection. Therefore, serial imaging of the aorta is mandatory. Magnetic resonance imaging (MRI) is the test of choice for follow-up imaging, although CT is an acceptable alternative. The current practice is to have imaging performed at 1, 3, 6, and 12 months after discharge, and annually thereafter. Surgery is indicated for aneurysmal expansion.
In our opinion, management of all patients with dissection, hematoma, and ulcers should include aggressive blood pressure lowering, goal LDL < 70, and antiplatelet therapy.
Vascular medicine specialists are ideally suited to provide long-term follow-up for patients with aortic dissection given their expertise in cardiovascular risk modification.