39 Discogenic Pain, Internal Disc Disruption, and Radicular Pain
This chapter concerns the diagnosis of spinal pain; diagnosis being the cornerstone of a doctor/patient relationship. Various spinal interventions will be discussed in following chapters. Each intervention requires particular knowledge and technical ability. Unfortunately, knowledge of the indications for these procedures and more importantly, their technical performance, have often fallen short of the mark—even in scientific publications.1,2 This has lead to a persistent skepticism about their efficacy. This fact is most evident in the development of radiofrequency neurotomy (RFN) treatments, and in subsequent meta-analyses and systematic reviews that gave equal credence to results, whether or not the technique was accurate.3
Social and Economic Dimension of Spinal Pain
Low back pain (LBP) is a major health problem particularly in industrialized countries,4 affecting approximately 60% to 80% of the adult population at some stage5–7 and about 6% of people each day.6 LBP affects up to 80% of the working population during their lifetime and is the second most common reason for physician visits,8 and for work disability.9 Although LBP is typically self-limiting,10 it is still associated with substantial health care costs and absenteeism from work.4,11,12 Neck pain is also extremely common with a lifetime prevalence of 70%, a 1-year prevalence of 40%, and a point prevalence of 10% to 20%.13 Generalized musculoskeletal pain is also common: in one study of kitchen-hand workers it was present in 87%, and often present in multiple sites. In this study, neck pain was present in 71%, LBP in 50%, and forearm/hand pain in 49%;14 73% had pain in at least two sites, 36% in four or more, and 10% in six to seven sites.14 In another study of 4006 workers from industrial and service companies, only 7.7% were free of regional pain of any description.15
In Australia, back problems are the most frequently seen musculoskeletal condition in general practice and the seventh most common reason for seeking care.16 In Australia, a 2001 survey found the point prevalence of LBP to be 26%, the 12-month prevalence 68%, and lifetime prevalence 79%.7 Only about 50% of the adult population experience low-intensity pain and low disability from it and another 11% experience high-intensity-pain but still low disability in a 6-month period.7 However, about 11% of the population experience high-disability LBP,7,17 and it is to this group that most resources are presumably directed.
LBP is a costly problem. In early 2000, the cost of headache, LBP, arthritis and other muscle and joint pain to U.S. employers was more than $60 billion per year (these costs may have been underestimated because lost productivity among workers affected by a coworker’s diminished productivity was not taken into account).18 The majority of these costs (77%) related to reduced performance rather than work absence (workers who experienced lost productive time from a pain condition lost a mean of 4.6 hours/week). Workers who reported arthritis or LBP had mean lost productive times of 5.2 hours/week. It was established that these pains were directly associated with a 13% loss in productive time. Headache was the most common (5.4%) pain condition resulting in lost productive time. It was followed by LBP (3.2%), arthritis pain (2.0%), and other musculoskeletal pain (2.0%).
Furthermore, the total health care expenditure only for LBP in the United States is even more alarming. When studied in 1998, total health care expenditures incurred by individuals with LBP in the United States reached $90.7 billion and total incremental expenditures attributable to LBP among these persons were approximately $26.3 billion. On average, individuals with LBP incurred health care expenditures at 60% higher than people without LBP.19 The lead researcher, Xuemei Luo, put these figures into the perspective of the U.S. economy by noting: “The total $90 billion spent in 1998 represented 1% of the U.S. Gross Domestic Product, and the $26 billion in direct back pain costs accounted for 2.5% of all health care expenditures for that year.”
The largest proportion of direct medical costs for LBP is spent on physical therapy (17%) and inpatient services (17%), followed by pharmacy (13%) and primary care (13%).20 However, indirect costs, especially resulting from lost work productivity, outweigh other costs substantially.20
Nonspecific Spinal Pain
Careful history and examination may at least be helpful in determining that the pain comes from the spine and whether or not it is caused by a red-flag condition. If clinical examination produces pain, it is likely that the pain derives from the spine. As a corollary, the absence of any painful restriction of movement on clinical examination should alert the clinician to the possibility of distant referred pain and such a negative finding is, in itself, a “red-flag sign”.21 Radiologic techniques are not helpful in resolving whether or not regional spinal pain originates from a spinal structure at all; imaging can exclude only red-flag or exotic causes of pain.
Numerous studies on all imaging modalities used in the detection of morphologic changes in symptomatic and asymptomatic populations have failed to find significant differences that can be considered useful in any individual presentation of NSSP.22–46 Astoundingly, this well developed scientific fact has still not penetrated into standard health care practice; consequently, certainly the public and regrettably, some heath practitioners, remain misinformed about the relevance of technology as it applies to NSSP. The perceived contribution of the disc to spinal pain has been skewed by inappropriate use of labeling in the field of radiology.47 The label degenerative disc disease (DDD) pervades imaging reports and the scientific literature, yet there is no reasonable evidence to confirm DDD as being of any particular relevance to any single manifestation of NSSP. DDD is not a legitimate label for a patient with NSSP.
NSSP concentrates along the spine; early studies on pain referral patterns have shown that all of the innervated back structures can produce local pain with or without more distant referred pain.48–54 When the pain is concentrated in the lumbar spine, it can be called nonspecific low back pain (NSLBP); in the cervical region, it is called nonspecific neck pain (NSNP). These terms are synonymous and equally as useful as terms such as idiopathic back (or neck) pain and low back (or neck) pain of unknown origin.
Referred Pain
Referred pain can theoretically derive from any locally innervated spinal structure. The mechanism for referred pain is convergence.55 Somatic referred pain is pain evoked by the stimulation of the peripheral endings of nociceptive afferent fibers and is perceived in an ambiguous site due to the phenomenon of convergence when these afferents converge on second-order or third-order neurons in the central nervous system that happen also to receive afferents from the region to which the pain is referred.56 Under those conditions, and in the absence of additional sensory input to clarify the situation, the brain is unable to identify the source of the pain accurately, and attributes it erroneously to the entire area subtended by the common neurons.57,58 Ambiguity as to the source of information arises, either or both, because the painful structure is not densely innervated, and the central pathways along which the information is relayed are not highly organized somatotopically.59 NSSP is, therefore, different to radicular pain. Hence, lumbar ZJ pain,60 lumbar disc pain,61–63 and sacroiliac joint pain64 are described very differently to lumbar radicular pain.
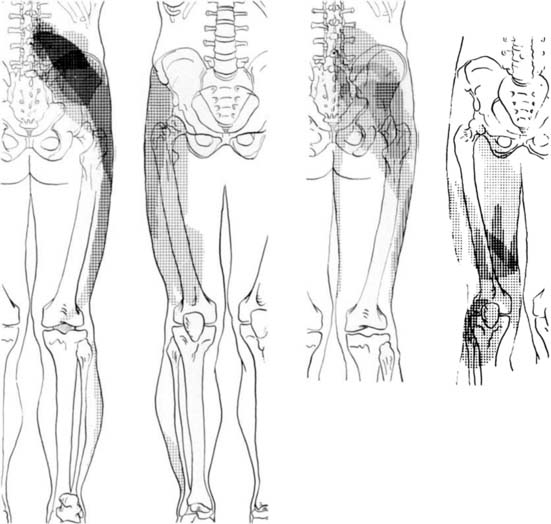
Information about referred pain from deep somatic structures has arisen from numerous important studies that identified the nature and spread of referred pain.48–54 Experiments on deep somatic referred pain by Kellgren52 in 1939 and Feinstein44 in 1954 showed that pain from deep somatic structures can be felt not only locally but also in distant areas; pain from deep lumbar structures refers into the legs as far as the feet; similarly pain from deep cervical structures refers into the arms and hands (Fig. 39-1). Later Hirsch and colleagues placed needles into various lumbar structures in people with LBP and reported that the disc was the most sensitive area for LBP.54 In this experiment, needles were placed into one or both of the lower two discs and into the ZJs, followed by the ligamentum flavum and the posterior ligamentous structures. When the disc was injected with 0.3 mL of saline a deep aching occurred across the low back. When the ZJ was injected, also with 0.3 mL saline, the ache spread also into the buttocks and lateral hips. Additionally, deep somatic pain felt three dimensional; it was described typically as deep and aching, but other terms used included gripping, boring, crampy, and lumpy.48 Subsequently the pain from lumbar PD, in which intradiscal pressures probably rise above that used by Hirsch and colleagues, has been shown to be experienced as central low back pain that can also spread diffusely into the legs,65 and certainly below the knees.66 From these studies, various referral pain maps have been created, demonstrating that segmental referral patterns overlap substantially, and structures at one segmental level have similar pain referrals to other structures at the same level.67 Thus, although the site of pain may be a clue to a particular spinal segment, it is not a pointer to the specific anatomic origin of pain.
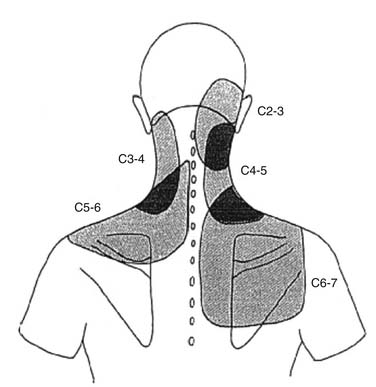
Similar patterns exist in the cervical spine. Cervical ZJ pain, as ascertained by anesthetic blocks rather than provocation, extends beyond the local region to the ipsilateral occiput, shoulder and/or periscapular region (Fig. 39-2).67 Cervical disc pain, as ascertained by PD, can spread into the thoracic spine and the arms including the forearms, hands, and fingers.68–70 Cervical PD at any level produces local neck pain that is unilateral as often as it is bilateral, with referral patterns as follows: C2-3 suboccipital and facial; C3-4 suboccipital, trapezius, anterior neck, face, shoulder, interscapular, and upper limb; C4-5 shoulder, interscapular, trapezius, extremity, face, chest and suboccipital; C5-6 trapezius, interscapular, suboccipital, anterior neck, chest and face; C6-7 interscapular, trapezius, shoulder, extremity and suboccipital; and C7-T1 interscapular (Fig. 39-3).71 Thus, cervical referred pain includes headache as well as arm, chest, shoulder, and thoracic pain.
Radicular Pain
NSSP and radicular pain can extend to the limbs; it is therefore important to ascertain which type of pain is more likely because each must be considered differently from clinical and pathophysiologic perspectives (Fig. 39-4).72 Radicular pain is not the same as referred pain.74 Radicular pain is a particular type of neurogenic pain caused by direct injury to a sensory nerve root or dorsal root ganglion of a spinal nerve.74,75 Radicular pain is sometimes accompanied by objective signs of deficit or loss of neurologic function in a segmental distribution as a result of conduction block and it can coexist with spinal or somatic referred pain.74,75
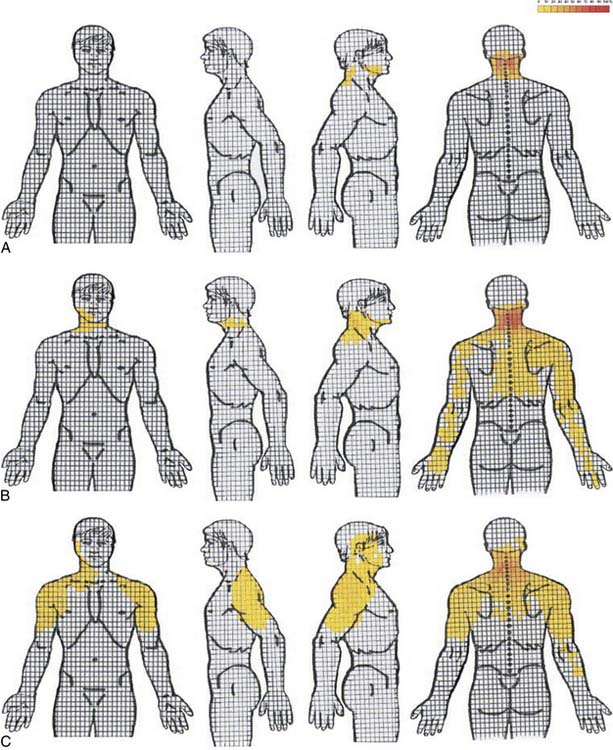
Cervical radicular pain tends to be deep, severe, aching pain, and as such is different from lumbar radicular pain.74 Cervical radicular pain is experienced predominantly in the upper limb and shoulder girdle but when it occurs in the limb its distribution does not correspond to dermatomal maps of sensory deficit due to cervical radiculopathy.73 Cervical radicular pain from the C6, C7, and C8 nerve roots is felt in the arm with pain extending into the forearm and hand (Fig. 39-5).75 However, limb pain can also occur in referred pain, and because cervical radicular pain is uncommon, with an annual prevalence rate of 0.083% in one large population study,76 deep aching arm pain is much more likely to be somatic referred pain than is cervical radicular pain.
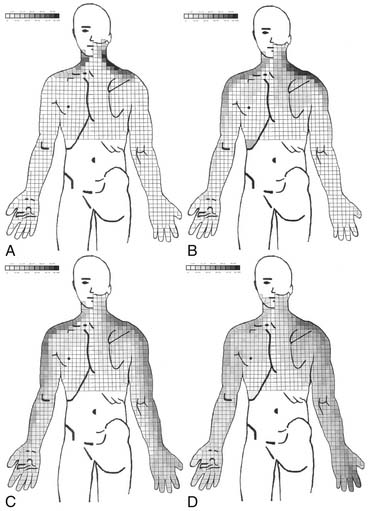
Figure 39-5 Percent occurrence of symptom provocation per bit for C4 to C7 nerve roots. A, C4; B, C5; C, C6; D, C7.73 Note that the figures depict anterior and posterior views.
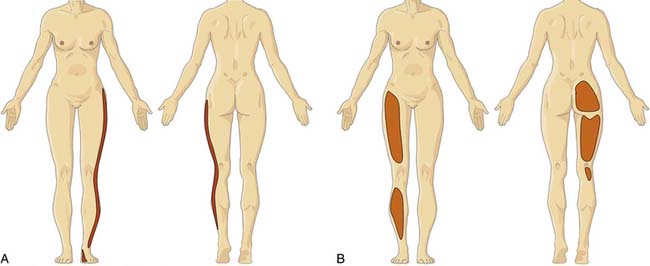
In contrast, lumbar radicular pain is classically an intense narrow band of lancinating, sometimes burning pain that refers down the limb, and often to the foot.77 The leg pain is typically more prominent than any LBP (indeed, LBP frequently is absent), and the leg pain tends to concentrate distally. Coughing, sneezing, straining at the toilet, and lifting will all classically exacerbate radicular pain, but such aggravating features are not specific to radicular pain. Pain is not limited to the dermatome and it may also be experienced in deep tissues innervated by the nerve.78
Concepts: Discogenic Pain, Internal Disc
As at least the outer one third of the annulus fibrosus (AF) is innervated,79–81 the intervertebral disc is considered to be a possible source of pain that is otherwise labeled NSSP. There are five types of nerve terminations found in the lumbar disc: these have various morphologies and include simple and complex free nerve endings that concentrate particularly in the lateral disc, with a smaller amount posteriorly and the least amount anteriorly.79 Discogenic pain (DP) is pain that is considered to arise intrinsically from the disc. DP is frequently bandied about and assumed as a diagnosis, but the plain fact is that it is a diagnosis that cannot be substantiated. With the use of PD, the entity IDD has been defined according to specific diagnostic criteria.82 IDD is considered to be a subtype of DP.
Is There a Relationship Between Disc Degeneration and Discogenic Pain?
Cellular function within the disc is mediated by at least five major factors: genetics, nutrition (diffusion of nutrients and oxygen across the disc matrix), cell function regulation (via IL-1, TNF-α, and TNF-β), age and senescence, and mechanical loading.83 The contribution to DD by genetic factors is highly significant; it may be as high as 80% in the cervical spine,84 and general heritability for DD ranges from 29% to 80% in different regions of the spine.84–86 In the lumbar spine, the genetic contribution is between 29% and 54%, with environmental influences of about equal importance.87 For example, smoking has a moderate influence on the prevalence of DD,87 presumably due to its effects on disc nutrition. This emphasizes that DD is not primarily or significantly caused by aging88 or by mechanically induced “wear and tear” processes.85,89
Studies with various imaging modalities on symptomatic and asymptomatic populations further emphasize that DD does not imply NSSP. In the cervical spine, radiologic DD is present in 13% of men and 5% of women during the third decade, in 85% to 90% of the population by the sixth decade, and nearly 100% by the age of 70 years.90 It occurs most commonly at C5-6, C6-7, and C4-5, respectively.42,91 In people aged 60 to 65 years without neck pain, about 95% of men and 70% of women have at least one degenerative change on their cervical spine plain radiographs.42 Lumbar degeneration, defined as a grade of ≥2 by the Kellgren-Lawrence scale; is present in Japanese females in 9.7% of the ≤ 39 age group, in 28.6% of those 40 to 49 years, in 41.7% of those 50 to 59 years, in 55.4% of those 60 to 69 years, in 75.1% of those 70 to 79 years, and 78.2% of those ≥ 80 years; and in Japanese males in 14.3% of the ≤ 39 age group, in 45.5% of those 40 to 49 years, in 72.9% of those 50 to 59 years, in 74.6% of those 60 to 69 years, in 85.3% of those 70 to 79 years, and 90.1% of those ≥ 80 years.92 Although plain radiographic changes, including vertebral end-plate changes, disc space narrowing, spondylolisthesis, spondylolysis, sacral lumbarization, wedge vertebra, a sagittal diameter of less than 12 mm and abnormal lumbar lordotic angle,44,93,94 have some predictive value for LBP, the relationship is mild at best,95 and their detection is largely not helpful in the management of NSLBP because such changes occur frequently in the asymptomatic population.96 Although disc space narrowing at 2 or more levels from L1-2 to L4-5 shows the strongest radiologic relationship with LBP,97 similar comments apply. As a consequence, plain radiographs should not be ordered unless there is suspicion of a red-flag condition.98–101
The relevance of CT scanning is similar to plain radiographs; it is an excellent test for some red-flag conditions, demonstrates DD well, but it is not helpful in the detection of DP or ZJ pain. CT is better than MRI in detecting ZJ spondylitis,102 but this is of no particular clinical relevance. A newer technology, F-PET/CT (fluoride positron emission tomography with addition of CT) is more likely to be positive in symptomatic patients,103 but only time will tell if it has satisfactory validity in detecting a truly painful structure.
Diagnosis: Discogenic Pain and Internal Disc Disruption
The Diagnosis of Discogenic Pain Using Specific Nerve Blocks
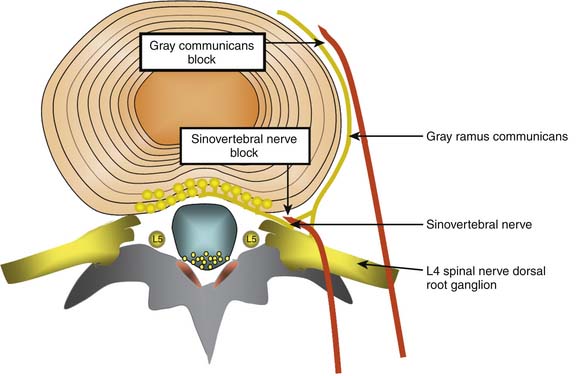
Lumbar discs are innervated by the lumbar sinuvertebral nerves, and branches of the lumbar ventral rami and gray rami communicantes (Fig. 39-6).104–106 The posterior and posterolateral portion of each lumbar disc is innervated by a branch of the ventral ramus arising just lateral to the intervertebral foramen and by a branch of the gray ramus communicans just before it connects with the ventral ramus.106 The sinuvertebral nerves also innervate the posterior longitudinal ligament; the gray rami communicantes also innervate the lateral disc and the anterior longitudinal ligament,104,106 and in rats the DRG innervates other lumbar structures, such as, lamina, spinous process, back muscle fascia, and skin.107 The innervation by the gray rami communicantes is not a direct sympathetic innervation;106 it has been postulated that somatic and afferent fibers from lumbar structures use the gray rami as transmission pathways only.104,108,109 The rami communicantes branch from the spinal nerves just after they enter the intervertebral foramina, and then run anteriorly along the inferior third aspect of the vertebral body where they connect to the sympathetic trunk before branching to the lateral and anterolateral aspects of the discs above and below.104,110,111
Various studies have been used to support or refute the utility of blocking the innervation of the disc.112–115 Although the nerve supply of the disc is nonspecific, if a local anesthetic block aimed at these nerves totally eradicated pain under controlled conditions, it might be possible to devise a treatment directed at the nerve similar to that performed for medial branch RF neurotomy. If this was the case, the interpretation would be that the pain was mediated by this particular nerve, not that the pain was necessarily discogenic.
The Diagnosis of Discogenic Pain or IDD Using Anesthetic Discography
The disc itself can be blocked by intradiscal injection. Analgesic discography (AD) and functional analgesic discography are refinements of the technique of PD aimed at increasing the utility of discography and, in particular, its specificity.116 However, the current definition of IDD does not include any statement about AD. There is no role for AD in discs with breaches through the outer lamellae (Dallas grade V) because pain reduction with leakage of any anesthetic agent into the epidural space could be a false-positive finding. This is the primary reason why there is at present no method for the diagnosis of DP in general; it is probable that discs with breaches in the outer lamellae can be a source of pain, but there is no valid method to make this connection. The problem with AD is that the anesthetic may not reach the painful radial tear owing to the dilution effect of contrast. If AD is to be used, it probably should be done directly via injection into the radial fissure.
The Diagnosis of IDD with Discography
Although lumbar DP cannot be diagnosed using imaging, there is a significant relationship between disc morphology as determined by discography, and clinical pain as determined by the subjective provocation phase of PD. As a consequence, a particular cohort of otherwise labeled NSLBP patients can be defined as having IDD,117 a specific subtype of DP.
Studies and reports on intradiscal therapies for putative DP have largely been drawn from patients diagnosed with IDD on the basis of PD. Discussion about the role and interpretation of PD can be found in Chapter 38. PD is used to confirm or deny the diagnosis of IDD, allowing for (1) enhanced ability to make a decision on interventional treatment—be it intradiscal therapy or spinal surgery; and (2) cessation of the search for other pain sources. The specificity of PD and, hence, the robustness of the diagnosis IDD has been questioned because of its propensity for false-positive findings.118–123 This can be minimized by careful patient selection; the risk of false positive findings is substantially diminished by selecting subjects with normal psychometric profiles who do not have pain in other regions.119,120 It would be folly to perform PD on a patient with fibromyalgia! The criteria for IDD are specifically applied to the lower lumbar spine (Table 39-1). There is insufficient research on other areas of the spine to consider that intradiscal therapies have any traction in the cervical and thoracic spine for such regional NSSP presentations. In the cervical spine the disc cannot be pressurized because fissures are present in normal adult discs.124 Nevertheless, intradiscal therapies have been trialed in other regions.
Table 39-1 Eligibility Criteria for IDD Treatment
| Chronic, disabling NSLBP |
| Failure to respond to noninvasive treatments |
| No red-flag conditions |
| No evidence of radicular or neuropathic pain |
| No psychological barriers |
| No greater than 25% loss of disc height |
| Criteria for IDD by provocation discography satisfied |
IDD, internal disc disruption; NSLBP, nonspecific low back pain.
This last point is likely to be highly significant. The histology of discs that have been diagnosed with IDD using PD is different for discs in patients without LBP that have been assessed by PD as negative but degenerate on MRI and for cadaver discs that are macroscopically normal; the major difference is that in IDD there is a chronic inflammatory reaction with variable blood vessel infiltration.129 More specifically, the AF loses its normal lamellar structure, is disorganized and disrupted, and the fibers are cross-fused; the NP is markedly fibrosed, inflamed, and infiltrated with blood vessels.129 Additionally, immunohistochemical staining shows strong connective tissue growth factor (CTGF) expression in IDD discs, weak expression in asymptomatic DDs, and no expression in adjacent control discs in patients with IDD.129
CTGF is the downstream effector mediated by transforming growth factor-β1 (TGF-β1).130 CTGF is “closely associated with the regulation of cell proliferation and differentiation and the fibrosis of tissues and organs, and can induce the in vivo expression of the gene involved with fibroblast extracellular matrix composition.”130 CTGF has a number of functions, one of which is the creation of some of the components of fibrosis by the production and accumulation of extracellular matrix.131 Healing of disc tissue is different to most other tissues because it is relatively avascular and takes place either from peripheral structures such as the outer AF and posterior longitudinal ligament,131 or via the end plate.132,133 Healing after injury to the AF or end plate is promoted and accompanied by vascular ingrowth, which stimulates vascular inflammatory reactions and the production of growth factors including TGF-β1 and CTGF.133 Aberrations of growth factor contribution to healing after injury to the AF or end plate are postulated to be a significant cause of DP.
The validity of the diagnosis IDD is predicated first on the methodology of the PD, and second on patient selection for the procedure. The International Spine Interventional Society mandates the protocol as follows:82
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree