Chapter 11 Disc Herniations
Injections and Minimally Invasive Techniques
 The diagnosis of a herniated disc starts with a complete history and physical examination. The addition of radiological studies should serve to confirm the diagnosis.
The diagnosis of a herniated disc starts with a complete history and physical examination. The addition of radiological studies should serve to confirm the diagnosis. Pain and neurological symptoms from a disc herniation are the end result of a combination of both chemical and mechanical factors.
Pain and neurological symptoms from a disc herniation are the end result of a combination of both chemical and mechanical factors. A disc herniation is one of the end results of a more chronic degenerative cascade that occurs with disc aging.
A disc herniation is one of the end results of a more chronic degenerative cascade that occurs with disc aging. It is important to understand the different types of disc herniations that exist—contained vs. extruded—and how they affect the response to interventions.
It is important to understand the different types of disc herniations that exist—contained vs. extruded—and how they affect the response to interventions. Success with percutaneous disc techniques depends on proper patient selection. Familiarity with MRI imaging and discography help in making the diagnosis of a contained disc herniation with an intact outer annulus fibrosis.
Success with percutaneous disc techniques depends on proper patient selection. Familiarity with MRI imaging and discography help in making the diagnosis of a contained disc herniation with an intact outer annulus fibrosis. An extruded disc herniation creates pain via both chemical and mechanical factors while a contained disc tends to create radicular pain via mechanical influences alone.
An extruded disc herniation creates pain via both chemical and mechanical factors while a contained disc tends to create radicular pain via mechanical influences alone. Epidural injections of steroids, TNF-α, and in rare cases use of oxygen-ozone therapy are the preferred modes of treatment of the chemical changes associated with extruded disc herniations.
Epidural injections of steroids, TNF-α, and in rare cases use of oxygen-ozone therapy are the preferred modes of treatment of the chemical changes associated with extruded disc herniations. The use of TNF-α inhibitors has shown promise for treating disc herniations but the more recent blinded clinical studies have been less favorable than the initial studies.
The use of TNF-α inhibitors has shown promise for treating disc herniations but the more recent blinded clinical studies have been less favorable than the initial studies. Percutaneous disc decompression techniques are viable treatment options for treating radicular pain secondary to the compressive mechanical forces that are exerted on nerve roots by contained bulging disc herniations.
Percutaneous disc decompression techniques are viable treatment options for treating radicular pain secondary to the compressive mechanical forces that are exerted on nerve roots by contained bulging disc herniations. All percutaneous lumbar disc decompression techniques are based on the same theory. The technical aspects of disc coblation, the lumbar decompressor, LASE, and PLDD all are similar in nature and require the same skill sets.
All percutaneous lumbar disc decompression techniques are based on the same theory. The technical aspects of disc coblation, the lumbar decompressor, LASE, and PLDD all are similar in nature and require the same skill sets. Chymopapain is the only decompressive disc treatment that can be used for contained and non-contained disc herniations so long as they are not extruded or sequestered.
Chymopapain is the only decompressive disc treatment that can be used for contained and non-contained disc herniations so long as they are not extruded or sequestered. All lumbar disc interventions have risk. Most notably they are associated with infections, bleeding, direct nerve trauma, and neurovascular injury. They should not be attempted for weak indications.
All lumbar disc interventions have risk. Most notably they are associated with infections, bleeding, direct nerve trauma, and neurovascular injury. They should not be attempted for weak indications. The performance of percutaneous disc decompression techniques requires at least a moderate degree of familiarity with intradiscal procedures, notably discography. This should be mastered by the provider before he/she attempts the more invasive intradiscal therapies.
The performance of percutaneous disc decompression techniques requires at least a moderate degree of familiarity with intradiscal procedures, notably discography. This should be mastered by the provider before he/she attempts the more invasive intradiscal therapies. Lumbar epidural steroid injections are the most commonly performed lumbar injections for disc herniations, but the efficacy of this procedure remains a point of controversy.
Lumbar epidural steroid injections are the most commonly performed lumbar injections for disc herniations, but the efficacy of this procedure remains a point of controversy. There is compelling evidence for the use of chymopapain in treating acute disc herniations, but its use has come under question secondary to rare but devastating complications in the past.
There is compelling evidence for the use of chymopapain in treating acute disc herniations, but its use has come under question secondary to rare but devastating complications in the past. A variety of lumbar percutaneous disc decompression procedures are available for use. The outcomes for each of them generally have been favorable in the treatment of contained lumbar disc herniations. However, more research is needed to identify the ideal candidates for a specific technique.
A variety of lumbar percutaneous disc decompression procedures are available for use. The outcomes for each of them generally have been favorable in the treatment of contained lumbar disc herniations. However, more research is needed to identify the ideal candidates for a specific technique.Establishing the Diagnosis
In 1934 the syndrome of “disc herniation” was born when Mixter and Barr1 first proclaimed that a posterior rupture of the intervertebral disc that allowed nuclear material to escape and compress the adjacent spinal nerve root(s) was a common cause of back and leg pain, a condition commonly known as sciatica.
Determining the presence of a disc herniation starts with a careful history. A herniated disc can lead to radiculopathy, radicular pain, or referred pain. Radiculopathy describes neurological changes such as numbness, weakness, and diminished reflexes with or without the presence of pain. Radicular pain is pain that typically follows a dermatomal pattern with or without the presence of sensory or motor deficits. Referred pain is pain that spreads into the lower limbs and is perceived in regions innervated by nerves other than those that innervate the site of noxious stimulation.2
Anatomy
The lumbar spine serves two basic and vital functions. The first is that it provides the structural framework that houses the spinal cord and spinal nerves. The second is that it provides a mechanical support for the entire axial skeleton. The lumbar spine is composed of five motion segments. Each motion segment consists of the vertebral body, intervertebral disc, pedicles, zygapophyseal joints, posterior lamina, and spinous process. These structural elements are supported by a vast array of ligaments that help maintain the stability of the spine (Fig. 11-1).
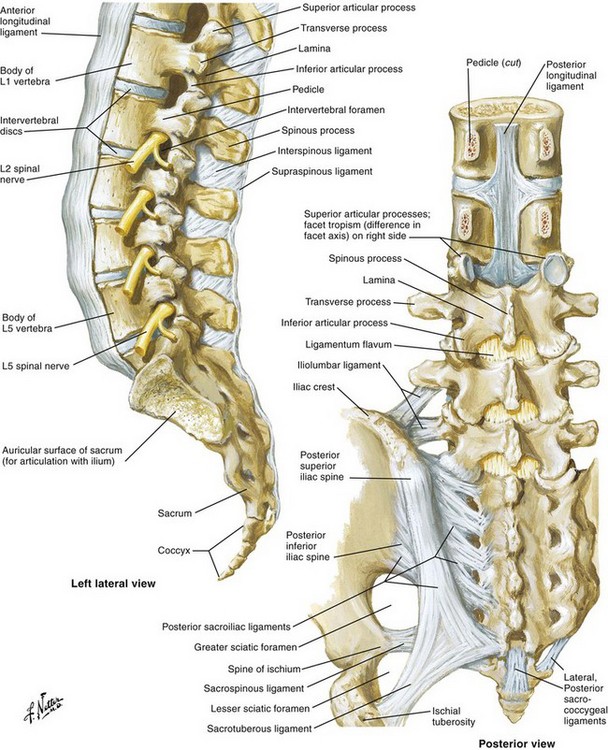
Fig. 11-1 Spinal anatomy.
Netter illustration from www.netterimages.com. ©Elsevier Inc. All rights reserved.
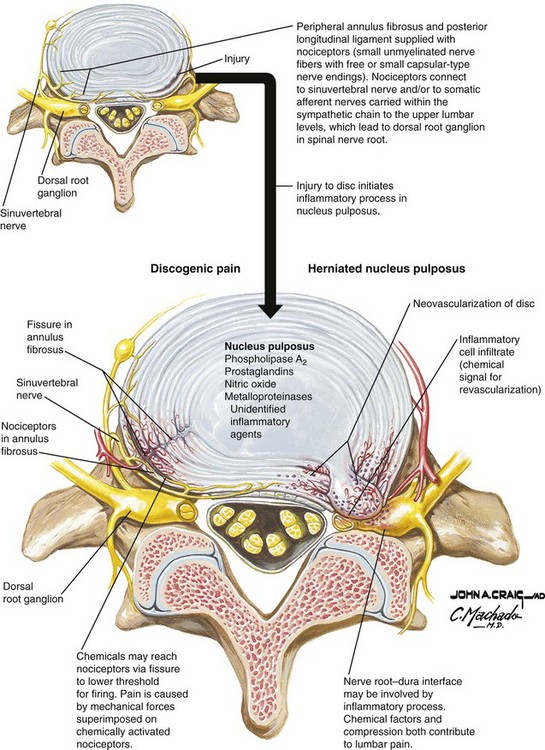
The normal disc is innervated in the outer one third of the annulus by fibers from the sinuvertebral nerve, which also supplies nociceptive input from the dural sac and nerve root sleeve. Branches of the gray rami communicantes have also been identified in the anterolateral annulus.3,4
As the disc degenerates and internal fissures develop, nociceptive fibers may invade into deeper layers of the AF, sometimes reaching the NP itself. These fibers travel along with vascular structures toward the center of the disc.5 Once the fissure has penetrated to the nucleus, inflammatory mediators contained within the NP now are exposed to nervous elements. This process, known as internal disc disruption, has been theorized as a major component of chronic low back pain.6
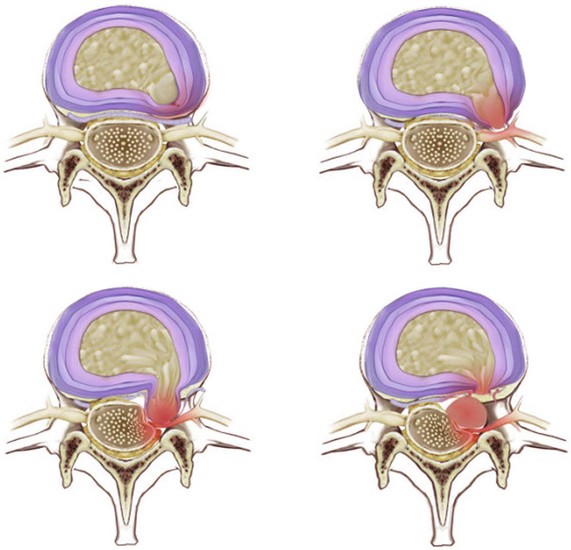
A gradual weakening of the outer annulus occurs with aging and endplate disruption. In the normal disc axial loads are distributed evenly across the AF and NP. However, in the case of internal disc disruption, the nucleus no longer evenly shares weight-bearing loads with the annulus. This may cause a generalized disc bulging that is evenly distributed across the annular fibers, or a unilateral bulging. Alone or in combination with other age-related changes (facet arthropathy, ligamentum flavum hypertrophy), this can lead to nerve root compression. Under high mechanical stress the outer annular fibers may disrupt entirely, allowing nuclear material to be displaced outside of the disc itself, resulting in an extruded or sequestered disc herniation (Fig. 11-2).7
Basic Science
The intervertebral disc is immunogenic. This is because, after embryonic development is complete, the avascular NP no longer has exposure to the immune system. Thus, when NP is introduced outside of the confines of the annulus, it is capable of inducing an autoimmune, inflammatory response.8
Several inflammatory mediators have been identified in the intervertebral disc in laboratory models designed to duplicate the chemical effects of disc herniation. These inflammatory mediators include phospholipase A2, prostaglandin E2, interleukin 1-α, interleukin 1-β, interleukin-6, tumor necrosis factor-α, and nitrous oxide (NO).9,10
Animal studies in which autologous NP and AF were placed on or even adjacent to nerve roots demonstrate that it is the nuclear material that induces the chemical radiculitis. The application of NP to nerve roots has been shown to induce axonal and myelin sheets alterations, increased vascular permeability, and decrease intraneural blood flow. Not surprisingly, studies evaluating antagonists to various inflammatory mediators have shown them to abate or prevent hyperalgesic responses in animal models.11,12
There are equally compelling studies involving the effects of mechanical compression. Direct compression of the nerve root both proximal and distal to the dorsal root ganglion (DRG), with or without extruded NP, results in pain-related behaviors in animal models. These pain-related behaviors are affected by a variety of factors, most notably apoptosis.13,14 Mechanical compression can produce apoptosis of the more severely injured neurons, which leads to retrograde transport of inflammatory mediators to the DRG, thereby inducing mechanical allodynia. This may be further exacerbated by diminished blood flow caused by edema and direct compression of the neuronal microvasculature. This explains why a disc bulge without extrusion can still produce neuropathic pain.
Imaging
MRI remains the modality of choice in diagnosing lumbar disc herniations. Its benefits include excellent resolution of the disc itself and the surrounding nerves, ligaments, and bony structures. The downside is that MRI is relatively sensitive in demonstrating degenerative changes, which can result in the detection of pathology in segments that are not pain generators.15
The nomenclature for describing disc abnormalities on MRI can be ambiguous. For example, there is no defined set of descriptors for grading or defining lumbar disc herniations. Perhaps more important, when considering whether percutaneous therapies are indicated, there is a need to establish whether or not a disc herniation is contained. MRI evaluations have been shown to be only 70% accurate in making an accurate diagnosis of a contained vs. noncontained disc herniation.16 Frequently discography can provide valuable information regarding whether or not a disc herniation is contained with the outer fibers of the annulus intact and pathology that is amenable to percutaneous interventions.17
For the purposes of this chapter, disc herniations are classified as contained and noncontained disc herniations and then divided into the following four categories (Fig. 11-3)7:
Epidural Steroid Injections (Interlaminar, Transforaminal, and Caudal)
The rationale behind epidural steroid injection (ESI) is that higher concentrations of corticosteroid are delivered to the inflamed nerve root(s) than with oral or parenteral routes, resulting in enhanced pain relief and reduced side effects. ESIs have been studied predominantly in patients with radicular pain (Table 11-1), which is most commonly caused by a disc herniation. There are three ways to access the epidural space: the caudal, interlaminar, and transforaminal approaches. The interlaminar and transforaminal techniques can be used in the cervical, thoracic, and lumbar spine. The caudal epidural space is accessed via the sacral hiatus and thus is reserved for lumbosacral symptomatology.
Table 11-1: Selecting Candidates for Epidural Steroid Injections
| Favorable Prognosis | Unfavorable Prognosis |
|---|---|
| Radiculopathy caused by herniated nucleus pulposus | Degenerative disc disease or spinal stenosis (short duration of benefit) |
| Short duration of symptoms | Pain >6 months in duration |
| Leg pain > back pain | Back pain > leg pain |
| Intermittent pain | Constant pain |
| Neuropathic descriptors (e.g., shooting, burning, electrical-like) | Nociceptive descriptors (e.g., aching, throbbing, dull) |
| Absence of psychopathology | Psychological overlay (e.g., somatization disorder, depression, poor coping skills, catastrophization) |
| Active lifestyle, good physical shape | Sedentary lifestyle, obesity, or deconditioning |
| Self-employed | On disability or with secondary gain issues |
| Young age | Older age, concomitant spinal pain generators |
| Isolated, single-level pathology | Multilevel pathology |
| No previous or previously successful interventions | Previous spine surgery or multiple failed interventions |
Indications/Contraindications
How many ESIs should be performed is also subject to debate. The practice of limiting injections to 3 in 6 months is not based on sound evidence in the form of prospective studies.18 Rather, the law of diminishing returns and the cumulative risk of steroid-related side effects and complications dictates an inverse relationship between the number of injections and the added benefit for each successive injection. Thus, although limiting the number of injections would appear to be prudent, the exact number and timing of injections need to be determined from clinical trials.
The need for repeat injections should be dictated by the patient’s response to the initial injection. If a patient obtains complete relief with one injection, a second injection can be done on an as-needed basis. If he or she has incomplete relief with the initial injection, a second injection at the same or different site can be repeated in as early as 1 week, although most people wait at least 2 weeks before repeating the procedure. No relief from the first injection warrants reevaluation; if a decision is made to repeat the ESI, either switching the route (i.e., transforaminal instead of interlaminar or intervertebral level) may be beneficial. There are no data to justify an automatic series of ESIs. Most interventionalists limit the use of ESIs to three or four in a 6-month period. The literature suggests that patients who respond to ESI usually require between one and three injections.18,19
Risk and Complication Avoidance
Epidural injections are subject to the same generic complications associated with any procedure such as bleeding, infection, and inadvertent puncture of the adjacent structures (e.g., the dura, kidneys, and blood vessels). Nevertheless, the rare complications that do occur can produce devastating complications such as meningitis, epidural abscess, and epidural hematomas.20
The complication that has received most interest of late is inadvertent spinal cord infarction after transforaminal ESI. There have also been at least 10 cases of this occurring after lumbar transforaminal ESI. Some cases have occurred at levels as low as S1. The most prominent theory as to the etiology of this complication is that there is inadvertent puncture of a spinal radiculomedullary artery. The largest of these vessels, the artery of Adamkiewicz, frequently projects into the lumbar spine as low as the L3 level. Unrecognized injection of particulate steroids into one of these arteries can cause occlusion of blood flow to the spinal cord, leading to infarction. Methods to reduce the risk of this complication include using continuous fluoroscopy or subtraction angiography during injection, minimizing manipulation of the needle, administering a nonparticulate steroid such as dexamethasone, injecting through a catheter inserted through the needle, and using a test dose of local anesthetic before injection.21
Epidural hematomas typically develop over a period of hours. The risk of epidural hematoma is less than 2 in 10,000 but increases in the presence of active anticoagulation, bleeding diathesis, and traumatic injection. Epidural hematomas classically present with severe low back pain and spasm along with progressive neurological deterioration. It is critical to recognize this complication within 8 hours because delayed intervention exponentially worsens prognosis.22
Although rare, there is a small risk of either bacterial meningitis or epidural abscess associated with ESI. The actual infectious risk from ESI is not well known since most literature is in the form of retrospective studies evaluating spinal anesthetics or continuous epidural catheters. The risk of meningitis secondary to intrathecal injection is estimated to be around 1 in 50,000.22
Anticoagulant Therapy
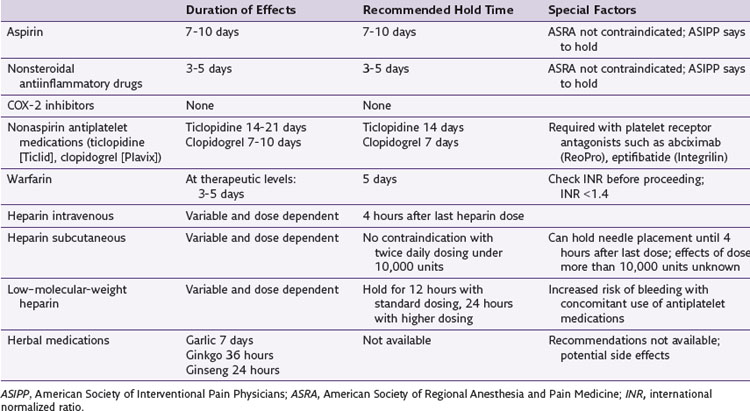
Regarding anticoagulant therapy, Raj and associates23 provided an exhaustive review for the American Society of Interventional Pain Physicians (ASIPP) of not just medication but patient and procedurally-related factors involved in assessing and minimizing the risk of bleeding complications.23 Horlocker and associates24 were more liberal in arriving at guidelines for the American Society of Regional Anesthesia and Pain Medicine (ASRA). Table 11-2 contains a simplified list of recommendations regarding anticoagulants. These do not address specific patient factors associated with bleeding.
Outcomes Evidence
A key shortcoming in evaluating the evidence for ESI is that many studies evaluating the efficacy of interlaminar injections were done without fluoroscopic guidance. Previous studies have demonstrated high rates (8.8% to 70%) of false loss of resistance for blind (without fluoroscopic guidance) ESIs, which may be higher in the cervical region.25,26 Even when the epidural space is successfully accessed, blinded injections may not deliver the medication to the area of pathology. In a study conducted in 50 patients with failed back surgery syndrome, Fredman and colleagues26 found that 5 mL of blindly administered injectate reached the targeted area only 26% of the time.
Multiple reviews have been written about the efficacy of ESIs. 27–30 These reviews are limited by reviewer bias (reviews conducted by people who perform epidural injections tend to be more favorable than those done by people who do not), the inclusion of studies with small sample sizes, serious methodological flaws, inadequate outcome measures, and heterogeneity with respect to route of injection and use of fluoroscopy. In one of the earliest reviews, Koes and associates27 illustrated the difficulty of properly evaluating the literature in a systematic review of 12 randomized clinical trials with disparate methodological qualities, half of which were deemed positive. The primary care physicians who conducted this review concluded that the benefits of epidural steroids, if any, seem to be of short duration. A similar review conducted 4 years later by a French task force of rheumatologists determined that 8 of 13 randomized studies demonstrated no measurable benefit.31 The authors of this analysis concluded that no determination could be made regarding the efficacy of epidural steroids for sciatica. The main weaknesses in the studies analyzed in these reviews were that none used fluoroscopic guidance and all used an interlaminar approach, which is probably clinically inferior to transforaminal ESIs.
In recent European guidelines for the management of low back pain, Airaksinen and colleagues32 concluded that epidural corticosteroid injections should be considered only for radicular pain, if a contained disc prolapse is the cause of the pain, and if the corticosteroid is injected close to the site of pathology. They further noted that injections should be fluoroscopically guided toward the ventral epidural space. These recommendations are in direct contrast with those outlined in a recent report by a subcommittee of the American Academy of Neurology that concluded that lumbosacral ESIs for radicular pain do not improve function, provide long-term pain relief (>3 months), or obviate the need for surgery.33 The authors found insufficient data to draw a conclusion for cervical ESI.
Recent systematic reviews by Abdi and associates28 and Boswell and associates34 reached different conclusions. The authors found strong evidence for short-term pain relief and functional improvement for lumbar transforaminal, cervical interlaminar, and caudal ESI and moderate evidence for long-term relief. The evidence supporting lumbar interlaminar injections was strong for short-term improvement but limited for long-term benefit.
One small randomized controlled study conducted by an orthopedic group found that lumbar TFESIs decreased the need for decompression surgery for up to 5 years since most of the patients who underwent lumbar TFESI elected not to undergo decompression surgery.35
Fluoroscopic guidance and method of injection (interlaminar vs. transforaminal) appear to account for most of the heterogeneity among the systematic reviews. The reviews with heterogeneity among methods of injection did not find a clinical benefit for the procedure,26,33 whereas those with stratified trials based on the method of injection found evidence to support transforaminal and caudal ESIs performed with fluoroscopic guidance.28,34 Considering that transforaminal and caudal ESI more reliably deliver medication to the ventral epidural space, this finding is not surprising.25 Transforaminal injections have also been found to be clinically superior to interlaminar ESI in two head-to-head comparisons.36,37 Transforaminal ESIs appear to afford better and longer-lasting relief than interlaminar and caudal ESI; however, the added benefit must be balanced against the higher risk.
TNF-α Inhibitors
Cytokines play a prominent role in the development of radiculopathy. Consequently there has been growing interest in using cytokine inducers and inhibitors for the treatment of the pain from herniated discs. Cytokines known to be involved in the pathological changes associated with herniated discs include tumor necrosis factor–alpha (TNF-α), interleukin (IL)-1, IL-6, and IL-8. After disc injury, IL1-β promotes matrix degeneration, and the administration of an IL-1 antagonist has been shown to prevent subsequent disc degeneration.38 TNF-α inhibitors appear to be involved in promoting thrombus formation, intraneural edema, and reduced nerve conduction velocity.39 However, the preemptive administration of TNF-α inhibitors in experimental models of herniated discs have been shown to prevent pain behaviors and reduce neurodegeneration.40