The Food and Drug Administration has approved everolimus for treating a variety of cancers. Everolimus inhibits signaling in the PI3K/AKT/MTOR pathway, which is critical for cell growth and angiogenesis. It can also suppress insulin output through AMPK/JNK/FOXO signaling. Here, we present the first case, to our knowledge, of everolimus-induced diabetic ketoacidosis and subsequent hypertriglyceridemia-induced pancreatitis in a cancer patient.
A 46-year-old woman with metastatic breast cancer presented to the emergency department (ED) of a comprehensive cancer center with the chief complaint of severe epigastric abdominal pain for 1 day, associated with nausea without vomiting. She had received a diagnosis of metastatic estrogen-receptor-positive breast cancer 1 year ago. After standard therapy, her cancer remained active, and she began receiving everolimus (10 mg daily) and continued receiving anastrozole (1 mg daily). She had a medical history of dyslipidemia that was well controlled with ezetimibe-simvastatin (10 to 80 mg daily) and gemfibrozil (600 mg twice daily). Her lipid profile was normal before everolimus was started. She had no history of diabetes mellitus, and her baseline glycohemoglobin level was normal (5.6%). After 6 weeks of treatment, everolimus was discontinued because of hyperglycemia (268 mg/dL) and hypertriglyceridemia (>1,550 mg/dL). She began to have epigastric pain 6 days after stopping everolimus, and she presented to our ED the next day.
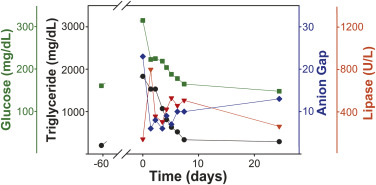
Her medical history included obesity, hypertension, hyperlipidemia, and status postcholecystectomy. Her physical examination result was unremarkable except for tachycardia (pulse rate 109 beats/min) and tenderness in the epigastrium. At the ED, pertinent laboratory findings included the following levels: serum bicarbonate 10 mEq/L (normal 18 to 30 mEq/L), glucose 315 mg/dL (normal 70 to 130 mg/dL), amylase less than 25 U/L (normal 23 to 85 U/L), lipase 140 U/L (normal 0 to 160 U/L), triglycerides greater than 1,550 mg/dL (normal 150 to 199 mg/dL), and urine ketone greater than 150 mg/dL (normal=not detectable). The calculated anion gap was 23. There was no evidence of infection, and serum lactate level was normal. Diabetic ketoacidosis was diagnosed and treated with intravenous insulin infusion. The glycohemoglobin level was 9.6%. Nausea and abdominal pain persisted, and repeated tests revealed a serum lipase level of 795 U/L, which was consistent with acute pancreatitis. In the absence of alcohol consumption, gallstone disease, or abdominal trauma, pancreatitis was likely due to everolimus-exacerbated hypertriglyceridemia. Her serial measurements of plasma triglyceride, glucose, lipase, and anion gap levels are shown in the Figure . She responded to treatment during 7 days and was discharged receiving metformin, glargine, fenofibrate, and atorvastatin.