FIGURE 1 Convergence of bottom-up and top-down factors at the RVM. The RVM receives information from the dorsal horn about peripheral input, and from higher structures such as hypothalamus, amygdala, anterior cingulate and prefrontal cortex, through direct and indirect (e.g., via the periaqueductal gray) pathways. It is thus a central node in a network that allows noxious input, cognitive and emotional factors to influence pain processing and sensation. Activation of ON-cells with suppression of OFF-cell firing during noxious stimulation forms a positive feedback loop.
DESCENDING CONTROL IS BIDIRECTIONAL AND MEDIATED BY DISTINCT CLASSES OF NEURONS
The idea of a supraspinal system that specifically modulates pain grew out of an observation first made over forty years ago, that electrical stimulation in the periaqueductal gray of the rat produces potent antinociception [57]. This revolutionary finding inspired work in a number of different laboratories, and ultimately led to the definition of a “pain-modulating network” with links in the periaqueductal gray and RVM. Stimulation at either site produces antinociception and inhibition of dorsal horn neurons. This network receives significant inputs from higher centers, including amygdala, hypothalamic nuclei, insula, and prefrontal cortex, providing a circuit through which nociceptive responsiveness can be influenced by cognitive and emotional factors, and matched to behavioral context [20,43,67]. This network also receives ascending nociceptive information from the dorsal horn, with input to both the periaqueductal gray and RVM [65,81]. Although the main output from this system is the RVM projection to the dorsal horn, the RVM also has ascending projections, with targets including amygdala and hypothalamus [28]. While the function or functions of ascending RVM projections are at present unknown, it is interesting to speculate that they provide an anatomical substrate through which brainstem systems could influence pain processing at higher levels, including cortex.
The periaqueductal gray-RVM circuit was long viewed as an endogenous “analgesia system,” and for several decades attention was focused on these areas as sources of descending inhibitory control. This system was implicated in both endogenous analgesia (for example, “stress-induced” analgesia) and in the actions of opioid analgesic drugs [21,66,77]. However, it subsequently became clear that descending control mediated by the RVM can be facilitatory as well as inhibitory [26]. The RVM modulates nociceptive tone on an ongoing basis, but the balance between inhibition and facilitation is dynamic, and can be altered in different behavioral, emotional, and pathological states. A facilitatory influence from the RVM is now known to be necessary for behavioral hypersensitivity in preclinical models of inflammatory and neuropathic pain [26,54,56]. A facilitatory role for the RVM has now been extended to incisional pain. Although an early study suggested that the RVM made no contribution to hyperalgesia induced by plantar incision [53], subsequent studies have reported that inactivation of the RVM reduces hyperalgesia in this model of post-operative pain [60].
Importantly, the influence of the RVM on nociception is not limited to control of nocifensor reflexes, but extends to the affective aspect of pain [8,29,37,72]. This includes ongoing pain following incision, where RVM block supports a conditioned place preference [9]. This finding indicates that the RVM contributes not just to enhanced intraspinal processing, but also to a state of aversion in animals subjected to surgery.
The physiology of RVM neurons is now reasonably well understood. RVM neurons can be divided into three classes based on changes in firing associated with noxious-evoked withdrawal responses [12]. “OFF-cells” are defined by a pause in firing associated with nociceptive withdrawal, and “ON-cells” by a withdrawal-related activation. The remaining RVM neurons, those without behavior-related changes in firing, are classified as “NEUTRAL-cells.” The validity of this physiological characterization of RVM neurons has been repeatedly confirmed in the distinct pharmacological profiles exhibited by the different cell classes [20]. None of the three cell classes expresses a unique neurotransmitter. Indeed many OFF-cells and ON-cells are GABAergic [74]. Although serotonin was long argued to be restricted to NEUTRAL-cells [46], further study has suggested that it may be found in ON- and OFF-cells as well [5,13].
The significance of the ON/OFF/NEUTRAL categorization of RVM neurons is that it ties neuronal activity directly to a behavioral index of pain, the withdrawal response, rather than to the noxious stimulus. That is, the behavior-related changes in firing that define ON- and OFF-cells are related not to the stimulus intensity per se, but to the decision to respond to the stimulus [4,32]. The implication is that the pause in OFF-cell firing and activation of ON-cells at the time of withdrawal are more closely related to the pain experienced than to the physical characteristics of the evoking stimulus.
The differentiation of these distinct cell classes has also allowed the pain-facilitating and pain-inhibiting output from the RVM to be dissected out. This is important because a change in pain measured behaviorally could reflect altered facilitation, changed inhibition, or a combination of the two. That is, hyperalgesia could be due to increased descending facilitation and/or reduced descending inhibition. The fact that ON-cells are activated in association with the withdrawal reflex suggested early on that these neurons had a facilitatory role, and the reflex-related pause in OFF-cell firing pointed to an antinociceptive role for this class [12]. Both proposals were confirmed in subsequent work that went beyond mere correlation, using a combined microinjection/single-cell recording approach in which a behaviorally relevant dose of various pharmacological agents was microinjected in lightly anesthetized, behaving animals while recording activity of an identified RVM neuron. In a series of studies, selective pharmacological activation of the ON-cell population was shown to be sufficient to produce behaviorally measurable hyperalgesia, whereas activation of OFF-cells led to hypoalgesia [25,50].
“BOTTOM-UP” RECRUITMENT OF THE RVM IN NORMAL AND POTENTIATED PAIN STATES
What do ON- and OFF-cells contribute to basal pain sensitivity? In naïve animals, both ON- and OFF-cell populations display fluctuating ongoing activity [1]. Selective block of ON-cell activity under these conditions has no measurable effect on nociceptive threshold, but reduces the magnitude of responses to noxious stimuli [24,33]. Presumed block of OFF-cells has at most a small hyperalgesic effect, indicating that the ongoing or “tonic” firing of OFF-cells has a modest influence on nociception under basal conditions [19,22,29]. This should not be surprising, since ongoing firing, if present, can be inhibited by the nociceptive GABAergic input that causes the OFF-cells to pause, thus permitting a behavioral response to the stimulus. The antinociception action of opioids and some other analgesic drugs is explained not be their effects on the ongoing discharge of OFF-cells, but by the fact that they act presynaptically to block this inhibitory input to OFF-cells so that the neurons remain active [21,23]. This concept, first proposed in the early 1980’s, was recently rediscovered and essentially confirmed in awake, head-restrained mice [27].
Acute insult
Severe pain, either pre-existing or resulting from the surgical procedure itself, is a strong risk factor for development of persistent pain [11,35]. In rodents, significant surgical insult, acute inflammation, and cutaneous administration of capsaicin all drive substantial increases in ongoing activity of the ON-cells (which, it should be noted, can mask any response to additional noxious stimulation). Additionally, OFF-cell firing is suppressed [3,6,36,42,62]. The increase in ON-cell discharge is mediated by NMDA and neurokinin-1 receptors [3,76]. This increase can be blocked selectively, using pharmacological tools. When this is done experimentally, behavioral hypersensitivity is reversed [36,76]. Thus, acute inflammation leads to a shift in the balance between ON- and OFF-cell outputs, such that ON-cells dominate (Fig. 2, Acute pain/inflammation), functioning as a positive feedback element that reinforces increased nociceptive processing at the level of the dorsal horn (Fig. 1). In essence, the increase in the ongoing activity of the ON-cells means that any primary afferent input enters a dorsal horn that is under a continuing pro-nociceptive modulatory influence [36,55].
It is worth noting that a similar shift in the balance towards ON-cell output can be seen in acute opioid withdrawal [34]. In this state, increased ON-cell excitability presumably reflects an opponent-process, compensatory response to the activation of OFF-cells and analgesia produced by the opioid. Thus, a shift towards ON-cell dominance can be triggered by analgesia, as well as by tissue damage and nociception. This shift is thought to contribute to “opioid-induced hyperalgesia” [69] and very likely plays a role in the long-lasting state of latent hypersensitivity observed in animals following opioid exposure [47,59]. Since the great majority of surgical patients receive an opioid analgesic at some point, consideration of how noxious insult and analgesic drugs interact to drive descending facilitation is an important topic for further study.
The shift in overall balance with acute injury so that ON-cells dominate the output of the RVM appears to be self-limiting. For example, following a localized injection of complete Freund’s adjuvant, the ongoing activity of ON-cells returns toward normal levels within hours, and is not maintained throughout the period of behavioral hyperalgesia [6,48]. The mechanisms underlying the apparent restoration of a “normal” ongoing balance between ON- and OFF-cells in the RVM are so far unknown.
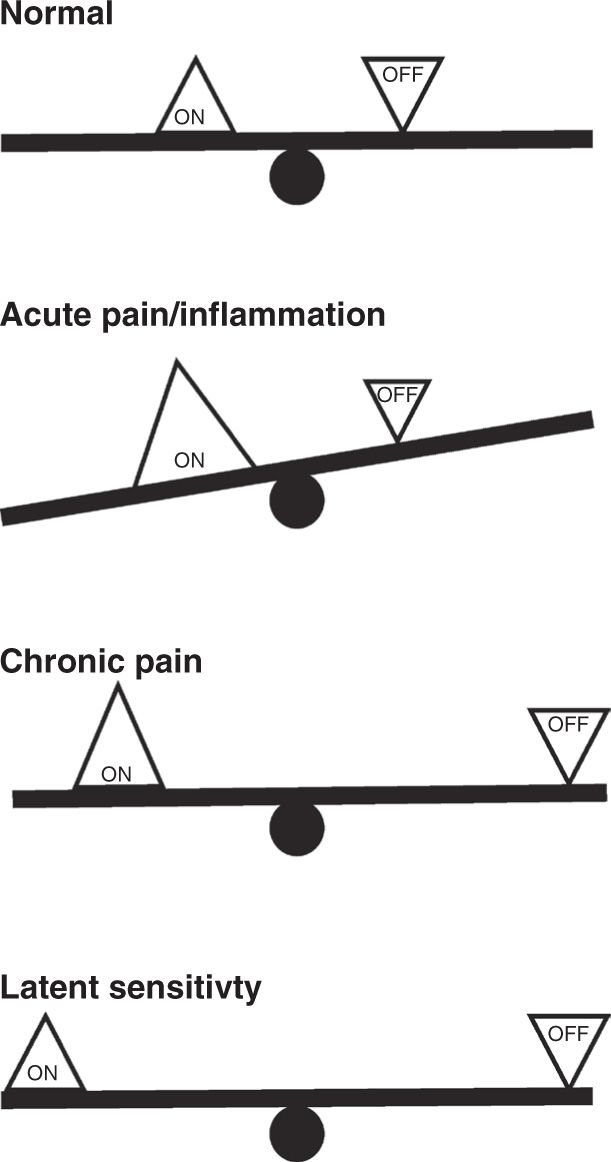
FIGURE 2 “Balance” of facilitatory and inhibitory outputs from the RVM in acute and chronic pain states. Using a seesaw analogy, we suggest that ON- and OFF-cells are essentially in balance under normal conditions. Both cell classes exhibit fluctuating activity, and neither output is dominant. During acute pain or inflammation, ON-cells are driven by noxious input while OFF-cell firing is inhibited. The facilitating output, the ON-cells, dominates. In chronic pain, potentiated inputs driven by sensitized primary afferents and dorsal horn circuitry are to some extent balanced by the inhibitory output. However, the system is less stable, as sensory inputs have moved further from the fulcrum. A smaller input is thus needed to trigger the behavior-related ON-cell burst and OFF-cell pause. Finally, in latent sensitization, the system returns to balance, but remains less stable.
Chronic State
The transition from acute to chronic pain is linked to significant physiological and molecular plasticity in the RVM. As inflammation becomes chronic, expression and function of NMDA, AMPA, neurokinin-1, opioid and trkB receptors are modified over a period of days [16–18,30,39,63], and glial cells are activated [61].
The physiology of ON- and OFF-cells in chronic pain states is quite distinct from that seen immediately after an acute insult or early in the course of inflammation. As noted above, the ongoing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







