KEY POINTS
In a patient with a dermatologic condition, observation and description of the lesions (morphology, distribution, and texture) are important for developing a differential diagnosis.
Mucous membranes (oral, ocular, nasal, genital, and perianal) should be examined in all patients.
The skin may provide clues to an underlying, life-threatening condition, such as endocarditis, graft-versus-host disease, bacterial and fungal sepsis, toxic shock syndrome, systemic vasculitis, or complications from the human immunodeficiency virus.
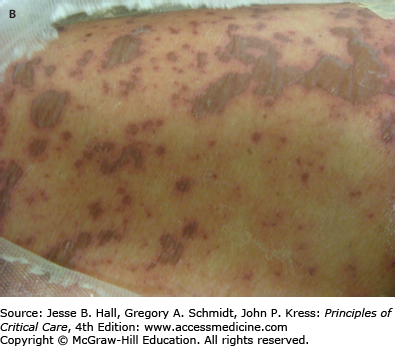
Drug-related dermatoses are prevalent in the intensive care unit. Clues to diagnosis include a rapidly developing eruption; generalized, symmetrical, predominantly truncal distribution; morbilliform, urticarial, or acneiform morphology; and accompanying pruritus.
Extensive skin disease can cause important fluid, electrolyte, and protein losses and predisposes the patient to life-threatening infections.
BASICS OF DERMATOLOGY
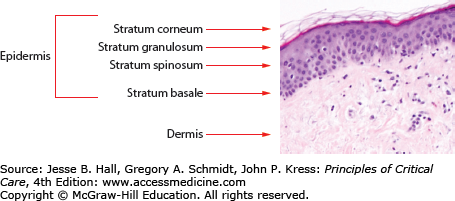
The basic anatomy of the skin is described in Figure 129-1. The skin is a complex organ whose major function is to provide a barrier against the environment. Other major functions include temperature regulation and sensation. The skin has three major layers: epidermis, dermis, and subcutaneous tissue. The outermost layer of the epidermis, the stratum corneum, is composed of dead, anucleate keratinocytes and serves as the first and major physical barrier. The stratum granulosum and stratum spinosum lie below the stratum corneum and are composed of keratinocytes in the process of differentiation. They are derived from the bottom layer of the epidermis, the basal cell layer. The epidermis is connected to the dermis by a complex of proteins and adhesion molecules in the basement membrane zone. Nutrients and products of metabolism are exchanged in the superficial and deep vascular networks located in the dermis. The dermis also contains nerve endings and supporting structures such as sebaceous glands, eccrine sweat glands, and hair follicles.
Alteration of any layer or structure of the skin can result in primary dermatologic disease. Often the skin is secondarily affected in underlying comorbid conditions and may serve as a window to internal disease processes. The stratum corneum can be damaged in the intensive care setting by tape, electrocardiographic leads, defibrillator devices, dry environments, pressure, or adhesives. Alteration may impair barrier resistance to infectious agents or allow passage of antigens to deeper layers of the skin. Preparing the skin for invasive procedures with topical solutions exposes the patient to potential sensitizers. Metabolically active cells in the suprabasal layers are susceptible to inflammatory and cytotoxic reactions from medications and toxins. Disruption in cell adhesion clinically manifests as blisters and may result from medications, toxins, pressure, extremes in temperature, or autoimmune diseases. Infections and inflammatory processes can occur at any level or in any structure, leading to conditions such as impetigo, folliculitis, cellulitis, fasciitis, or vasculitis.
When approaching a patient with skin disease, careful observation, palpation, and description are critical for developing a differential diagnosis. The morphology, or type, of lesion may be flat (macule), elevated (papule, nodule, plaque, cyst, vesicle, bulla, pustule, or hyperkeratosis), or depressed (ulcer or atrophy; Table 129-1). Shape, margination (well or poorly defined borders), and arrangement of the lesions are important. Color may be white (leukoderma or hypomelanosis), red (erythema), pink, violaceous (purple), brown (hypermelanosis or hemosiderin), black, blue, gray, or yellow. Particular attention should be paid to the distribution of the eruption (eg, localized, systemic, truncal, acral, unilateral, or intertriginous). Palpation will help determine consistency, temperature, mobility, tenderness, and depth of lesion. When various lesions are present, one should attempt to determine the primary lesion.
Basic Morphologic and Descriptive Terminology
| Macule | Flat lesion of variable size |
| Papule | Elevated lesion less than 0.5 cm diameter |
| Plaque | Elevated lesion greater than 0.5 cm diameter |
| Nodule | Elevated, palpable lesion greater than 0.5 cm diameter |
| Vesicle | Fluid filled lesion less than 0.5 cm diameter |
| Bullae | Fluid filled lesion greater than 0.5 cm diameter |
| Pustule | Pus-filled lesion |
| Ulcer | Depressed lesion with loss of epidermis and variable levels of dermis |
| Wheal | Evanescent pale-red papule or plaque |
Clinical history is essential. Cutaneous symptoms (pruritus, pain, tenderness, burning, or stinging) as well as systemic symptoms (fever, malaise, arthralgias, myalgias, etc) must be ascertained. Time course of the skin lesions should be determined, with particular attention to medication history (prescription and nonprescription, oral, topical, and alternative). Common diagnostic aids in dermatology are skin biopsy for hematoxylin and eosin or other special staining, direct immunofluorescence (DIF), or culture; potassium hydroxide preparation (for dermatophytic infections); mineral oil mounts (for scabies); Gram stain (for bacterial infections); fluid cultures; and Tzanck smears (for herpes simplex and varicella zoster virus infections).
DERMATOSES PRECIPITATED BY DRUGS
KEY POINTS
Morbilliform and urticarial eruptions are the two most common types of drug eruptions.
Less common reactions include pustular, bullous, vasculitic, lichenoid, and fixed drug eruptions.
Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) are three severe cutaneous drug reactions that need prompt recognition and initiation of treatment.
Therapy consists of withdrawal of the culprit drug, symptomatic relief with antihistamines ± topical or oral steroids, IVIG, and wound care when indicated.
The World Health Organization (WHO) defines an adverse drug reaction (ADR) as any noxious, unintended, and undesired effect of a drug that occurs at diagnostic, prophylactic, or therapeutic doses used in humans.1 This definition excludes untoward events due to noncompliance or errors in drug administration, therapeutic failures, intentional and accidental poisoning, and drug abuse. A meta-analysis of 39 prospective studies covering 32 years reported a 10.9% incidence of ADR in admitted hospital patients and a 4.7% incidence for patients admitted because of serious ADR.2 In addition, fatal ADR ranked “between the fourth and sixth” leading causes of death in the United States in 1994, exceeding deaths due to pneumonia and diabetes.2 The rate and severity of preventable ADRs in intensive care units (ICUs) are nearly twice that in non-ICUs.3
Cutaneous ADRs (CADRs) are the most common type of ADR and occur in 2% to 3% of hospitalized patients.4 The numbers of CADR may be higher in the ICU setting due to the critical and compromised nature of the patient compounded by the multiplicity of drugs. Several factors influence the probability of a drug producing an adverse reaction: the size of the compound (larger compounds are more likely to act as haptens), drug-drug interactions (altered metabolism and protein displacement), the route of delivery (intravenous administration increases the incidence of reactions), and patient factors such as renal function, alcohol use, hepatic function, and severity of concomitant disease.5,6 Antibiotics (eg, amoxicillin, penicillin, fluoroquinolones, sulfonamides, and cephalosporins) and nonsteroidal anti-inflammatory agents (NSAIDs) are the most likely medications to cause CADR. Antiepileptics (eg, phenytoin and carbamazepine) are also common causal agents. The following medications only rarely cause CADR: digoxin, acetaminophen, meperidine, aminophylline, diphenhydramine, bisacodyl, prochlorperazine, spironolactone, prednisone, thiamine, ferrous sulfate, atropine, morphine, insulin, and spironolactone.
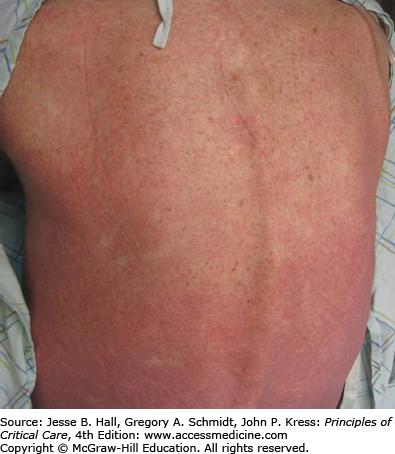
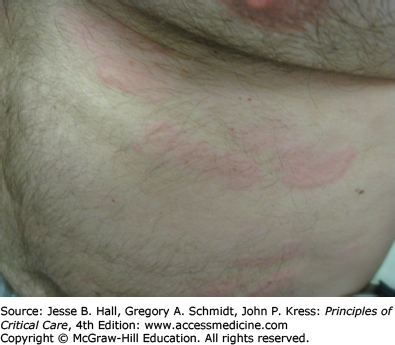
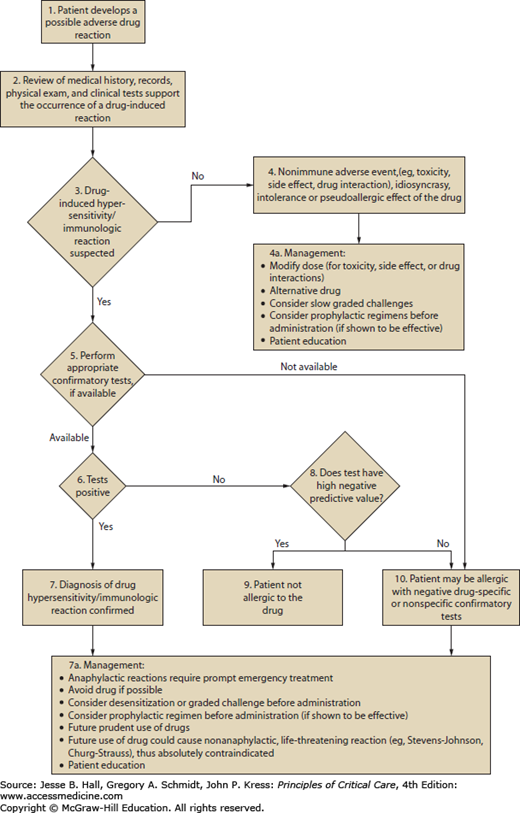
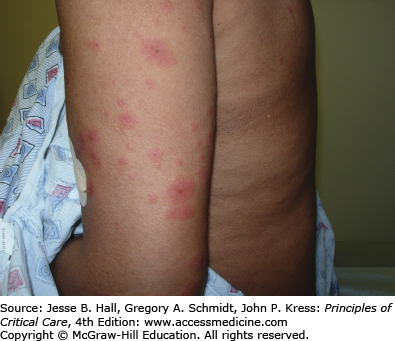
Cutaneous eruptions are the most frequent ADR in hospitalized patients. Morbilliform exanthems (Fig. 129-2) and urticaria (Fig. 129-3) are responsible for 95% and 5% of CADRs, respectively.7 Other less common drug-associated morphologies include lichenoid, photosensitive, vasculitic, and lupus-like patterns. Onset of the exanthem is usually within 1 week of administration, with the notable exceptions of antibiotics and allopurinol. Clues to diagnosis include (1) an eruption that develops very rapidly with an onset temporally related to the administration of a drug; (2) a generalized, symmetrical, predominantly truncal distribution; (3) an exanthematous (morbilliform), urticarial, fixed drug, or acneiform morphology; and (4) accompanying pruritus. Medical history, physical examination, and laboratory findings may provide clues, although an extensive laboratory workup is usually unnecessary for diagnosis (Fig. 129-4). Identifying the causative agent in the ICU setting may be problematic due to the concurrent administration of multiple drugs; hence, all medical records and family members or close contacts should be questioned. Table 129-2 outlines the information that must be obtained. With all this information in hand and knowing the reaction rate of various medications, identification of the cause of an eruption becomes more likely.
FIGURE 129-4
Algorithm for the management of adverse drug reactions. Adapted with permission from Executive summary of disease management of drug hypersensitivity: a practice parameter. Joint Task Force on Practice Parameters, the American Academy of Allergy, Asthma and Immunology, the American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. December 1999;83(6 pt 3):665-700.
Drug Eruption Checklist: Information to Be Elicited From the Patient’s Medical Records or Family
|
Despite the benign nature of the overwhelming majority of CADRs, it is important to evaluate for increasing liver or renal dysfunction and for signs suggesting progression to severe skin disease (Stevens-Johnson syndrome or toxic epidermal necrolysis). Signs indicative of serious skin problems include mucosal involvement, blistering lesions, and a positive Nikolsky sign (Table 129-3).
Indicators that an Adverse Drug Reaction May Become Serious
| Cutaneous Findings | Systemic Findings |
|---|---|
| Confluent erythema | High fever (>40°C) |
| Rash or edema involving the face | Lymphadenopathy |
| Tender skin lesions | Joint pain |
| Palpable purpura | Dyspnea, wheezing, hypotension |
| Necrotizing skin lesions | |
| Vesicles/bullae | Laboratory Findings |
| Positive Nikolsky signa | Abnormal liver function tests |
| Mucous membrane erosions | Lymphocytosis with atypia |
| Urticaria | Eosinophilia (>1000/mm3) |
| Tongue edema |
The most widely used classification scheme for ADR was devised by Rawlins and Thomson5 (Table 129-4). Type A reactions are the most common (80%) and can occur at any dose. Type B reactions occur in susceptible individuals (10%-15%) a few days after administration or with re-exposure, and are independent of dose. Type B comprises drug intolerance, idiosyncratic reactions, and allergic or hypersensitivity reactions. Allergic reactions can be categorized further into those that are mediated by drug-specific antibodies or drug-specific T lymphocytes. These include the four mechanisms identified in the Gell and Coombs classification: immediate, cytotoxic, immune complex, and delayed hypersensitivity.8
Classification of Cutaneous Adverse Drug Reactions (CADR)
|
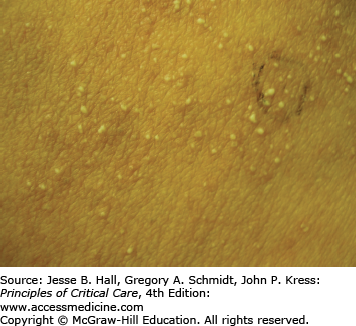
Type I (immediate) hypersensitivity reactions are mediated by immunoglobulin (Ig) E antibodies specific to the causative drug, found on mast cells and peripheral blood basophils, and occur more frequently with parenteral administration. They usually occur within 1 hour of drug administration, but may occur as late as 72 hours in the absence of prior sensitization to the drug. Type I hypersensitivity reactions often manifest as urticaria, angioedema, or anaphylaxis. Urticarial lesions are pruritic, erythematous or white, nonpitting, round or oval edematous papules or plaques surrounded by a clear or red halo, usually at different stages of formation (Fig. 129-3). Angioedema refers to the same pathophysiologic process as urticaria with transudation of interstitial fluid into the dermis or hypodermis. Anaphylaxis is a severe allergic reaction with systemic manifestations that comprise angioedema with laryngeal edema, bronchospasm, hypotension, diffuse erythema, hyperperistalsis, cardiac arrhythmias, urticaria, and pruritus. Drug-induced anaphylaxis occurs in 1 of 2700 hospitalized patients and is most frequently induced by β-lactam antibiotics (including penicillin), radiocontrast medium, intravenous anesthetic drugs, aspirin, other NSAIDs, and opiates. In the United States, the most common cause of anaphylaxis is penicillin.9 Treatment of urticaria consists of discontinuation of the offending drug and administration of oral antihistamine H1 blockers. These include diphenhydramine, hydroxyzine, and the nonsedating agents, loratadine, cetirizine, and fexofenadine. If anaphylaxis develops, emergency treatment is instituted with intramuscular or subcutaneous epinephrine, high-flow oxygen and airway management, intravenous diphenhydramine, steroids, fluids, vasopressors, and cardiopulmonary resuscitation, as needed (Chap. 128). Skin testing with the offending agent is usually positive in IgE-mediated reactions.
Cytotoxic (type II) reactions are mediated by IgG and complement, usually occur longer than 72 hours after drug exposure, and manifest as increased clearance of red blood cells and platelets by the lymphoreticular system. More rarely, they may manifest as intravascular destruction by complement-mediated lysis. Skin testing is not useful.
Type III reactions are serum sickness-like reactions, in which IgG or IgM immune complex deposition leads to diffuse tissue injury. Common clinical findings include fever, urticaria, angioedema, malaise, arthralgias (particularly of the hands and feet with swelling), lymphadenopathy, and occasionally nephritis or endocarditis, usually starting after 1 to 3 weeks of drug administration. There is an associated eosinophilia. Heterologous antisera, xenogeneic antibodies, and drugs such as penicillins, minocycline, bupropion, and propranolol are the most common triggers. Cefaclor, a second-generation cephalosporin, has also been reported to cause serum sickness-like reactions in adults, albeit less frequently than in children.10 Systemic steroids are often used to treat this reaction, although large-scale controlled clinical trials are lacking.
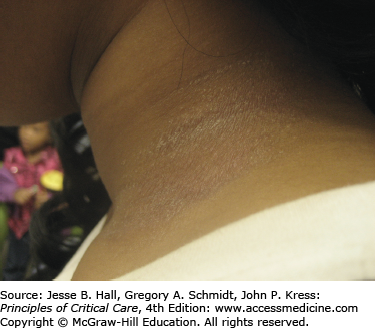
Type IV, delayed-type hypersensitivity reactions occur as a result of an immune reaction to a hapten-carrier complex. Under physiological conditions, drugs can bind covalently to a larger protein or peptide, forming stable hapten-carrier complexes which are then processed and presented on MHC molecules as immunogenic peptides. This leads to primary sensitization to the drug. After primary sensitization has occurred, an allergic reaction can be elicited by topical or systemic administration of the same or a structurally similar agent. Occasionally, a reaction may appear de novo after several days of contact with the offending agent. Allergic contact dermatitis (Fig. 129-5) is the most common type IV, delayed-type hypersensitivity reaction, usually caused by topically applied medications. A pruritic, erythematous, vesicular, scaly eruption occurs at the site of contact, which, with time, may become lichenified (thickened with accentuation of skin markings) related to rubbing or scratching.
Diagnostic tests for CADR may be useful to determine the causative agent, the type of reaction, and the prognosis. Skin testing may be performed by a prick or intradermal administration of the suspected drugs to determine the presence of drug-specific IgE antibodies (type I Gell-Coombs reactions). Penicillin, muscle relaxants, and barbiturates are amenable to this type of testing because their epitopes are known. Medicines that undergo significant metabolism and those with undefined epitopes cannot be tested. An in vitro test to study the presence of circulating drug-specific IgE antibodies is the radioallergosorbent test. Penicillin, insulin, thiopental, protamine, latex, chymopapain, and selected muscle relaxants can be tested by radioallergosorbent test, with variable consistency.
When ADRs are mediated by drug-specific T cells (type IV; eg, contact dermatitis), patch testing is a useful adjunct. It is performed by applying predetermined dilutions of the compound on intact skin for different periods and assessing for erythema, edema, or vesiculation, all indicative of specific T-cell reactivity. A skin biopsy aids in clarifying the pathophysiology of a reaction, for example, by the demonstration of immune complexes, leukocytoclastic vasculitis, or tissue eosinophilia. Blood eosinophil counts have been regarded as an indicator of ADR; however, recent studies have shown that this criterion carries a low sensitivity (22%-36%, depending on arbitrarily defined cutoff rates), making routine eosinophil testing unhelpful.11 Serum tryptase levels are useful in reactions caused by diffuse mast cell (anaphylactic or anaphylactoid) activation, especially when hemodynamic changes are present. Tryptase is a protease stored in the granules of mast cells and released with mast cell activation. Levels should be obtained within 2 hours of the anaphylactic episode. Serum tryptase concentrations are recommended over serum histamine concentrations due to the greater stability of tryptase.12
The clinical usefulness of in vitro diagnostic tests to detect the presence of drug-specific antibodies capable of eliciting basophil histamine release, lymphocyte blast transformation, or complement activation remains to be proven. These tests carry a low sensitivity, are currently used mainly as research tools, and therefore are not recommended for routine clinical use.
Despite the different proposed mechanisms, overlap is common, and one cannot infer the pathogenesis of an eruption by its morphology or causative drug alone. The diagnosis of a drug-related dermatosis encompasses a complex mental algorithm of probability, time and causality, alternative diagnoses, and plausibility.
Cancer patients may be affected by cutaneous reactions of diverse etiologies, including infections, paraneoplastic pemphigus, dermatomyositis, graft-versus-host disease (GVHD), nutritional deficiency, metastases, cutaneous neoplasms, radiation reactions, and administration of chemotherapeutic agents. Table 129-5 includes the most commonly encountered chemotherapy-induced dermatoses.
Chemotherapy-Induced Dermatoses
| Type of Reaction | Responsible Drugs | Treatment |
|---|---|---|
| Alopecia (anagen or telogen effluvium) | Vincristine, cyclophosphamide, doxorubicin, daunorubicin, dactinomycin, paclitaxel |
|
| Mucositis/stomatitis | Topotecan, methotrexate, fluorouracil, doxorubicin, dactinomycin, bleomycin, docetaxel, daunorubicin |
|
| Candidiasis | Vincristine, cisplatin, doxorubicin, daunorubicin |
|
| Extravasation reactions | Bleomycin, carboplatin, cyclophosphamide, doxorubicin, etoposide, paclitaxel, vinblastine |
|
| Hyperpigmentation (localized or diffuse) | Bleomycin,a cisplatin,b methotrexate,c cyclophosphamide, daunorubicin, doxorubicin fluorouracil, hydroxyurea, vinblastine, 5-fluorouracil |
|
| Acral erythema | Cytarabine, doxorubicin, fluorouracil |
|
| Lower extremity ulcerations | Hydroxyurea, methotrexate |
|
| Neutrophilic eccrine hidradenitis | Cytarabine, bleomycin, cyclophosphamide, cisplatin, topotecan, anthracyclines |
|
The most common CADR to chemotherapeutic agents is alopecia (hair loss), which may present as anagen (hair growth phase) or telogen (hair resting phase) effluvium.13 Anagen effluvium is usually caused by antineoplastic agents, which weaken the hair shaft, resulting in severe hair loss apparent 1 to 2 months after therapy. Telogen effluvium occurs secondary to acute physical or psychological stress, malnutrition, or drugs. Spontaneous regrowth with drug discontinuation is the norm for both types of alopecia. Treatments are rarely successful. The effectiveness of 2% topical minoxidil has been inconsistent.14,15
Adverse effects in the oral mucosa occur in 40% of patients as a result of direct drug toxicity or indirectly via effects on the bone marrow. Stomatitis is a frequent complication leading to erythema, edema, ulceration, pain, burning, and xerostomia. Healing occurs within 2 to 3 weeks. Treatment consists of adequate oral hygiene and the use of topical agents such as attapulgite, magnesium/aluminum hydroxide, diphenhydramine elixir, benzocaine, and viscous lidocaine.
Candidiasis, with its white, adherent velvety plaques, and varicella zoster virus may also affect the inside of the mouth. Herpes simplex usually affects the vermilion border of the lips, but in the immunosuppressed may cause indolent intraoral ulcers.
Intravenous administration of chemotherapeutic agents may result in extravasation into surrounding tissues. Severity depends on type, quantity, and concentration of drug,16 and disabling sequelae are uncommon. Agents may be irritants (eg, bleomycin, carboplatin, cyclophosphamide, doxorubicin, etoposide, fluorouracil, paclitaxel, or vinblastine), which cause sclerosis, hyperpigmentation, and tenderness along the vein, or vesicants (eg, bleomycin, carmustine, cisplatin, doxorubicin, etoposide, fluorouracil, melphalan, mechlorethamine, mitomycin, paclitaxel, vinblastine, or vincristine), which cause burning, necrosis, and ulceration. Treatment consists of discontinuing the infusion, elevating the affected extremity, and applying local heat or cold. Heat should not be applied when extravasation of vinca alkaloids occurs because this may cause ulceration.17 Surgical debridement and skin grafting may be needed in persistent ulcers. Antidotes are also recommended (Table 129-6).
Localized or diffuse hyperpigmentation is a common CADR, as a result of melanin, carotene, hemoglobin, or other pigment deposition. Characteristic gingival bands occur with cisplatin; horizontal hyperpigmented bands on blond hair (flag sign) with methotrexate; flagellate streaks of hyperpigmentation with bleomycin; serpentine supravenous hyperpigmentation primarily with 5-fluorouracil; pigmented nail bands with bleomycin, cytarabine, cyclophosphamide, daunorubicin, doxorubicin, fluorouracil, hydroxyurea, idarubicin, mitomycin, tegafur, or vinblastine; and mucosal hyperpigmentation with busulfan, fluorouracil, tegafur, doxorubicin, hydroxyurea, cisplatin, or cyclophosphamide.13
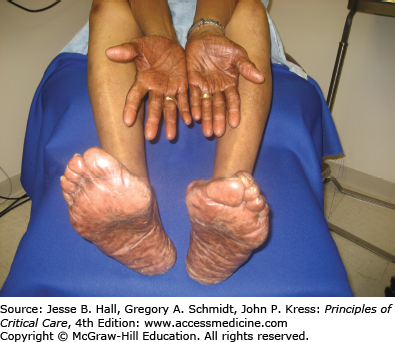
Another well-described CADR to chemotherapeutic agents is acral erythema (Fig. 129-6), occurring in 6% to 24% of patients,18 mainly those treated with cytarabine, doxorubicin, and fluorouracil. This painful eruption consists of diffuse erythema and edema on the palms and soles. This resolves with exfoliation within 4 weeks of discontinuing the drug. It needs to be differentiated from acute GVHD in the appropriate clinical setting. Treatment is supportive, with elevation, cold compresses, analgesics, and pyridoxine 150 mg each day.19
Cutaneous ulceration of the lower extremities is reported as a complication of long-term hydroxyurea,20 and rarely, high-dose methotrexate.21 Hydroxyurea-induced ulcers typically occur over the malleoli, are painful, and may be resistant to local wound care, topical or systemic antibiotic therapy, pentoxifylline, prednisone, hyperbaric oxygen, or Unna vascular boots. Ulcers heal over several months after cessation of hydroxyurea. The first case of lower extremity cutaneous ulceration in a patient without psoriasis receiving methotrexate was reported in 1998.21 Since then, several additional cases of methotrexate-related cutaneous ulcers have been reported.
Neutrophilic eccrine hidradenitis (NEH) is a disorder that results from a direct cytotoxic effect of chemotherapy on the eccrine glands. Erythematous papules and plaques occur on the trunk and the extremities within 2 weeks of starting chemotherapy. This condition is most often associated with the initiation of cytarabine in patients with acute myelogenous leukemia. Bleomycin, cyclophosphamide, anthracyclines, cisplatin, and topotecan have also been implicated. NEH typically resolves within weeks after cessation of chemotherapy, although it may recur in subsequent cycles. Dapsone is used to treat and prevent recurrences of NEH.22
Adverse reactions to anticoagulants can be localized or diffuse and mediated by various mechanisms. Unfractionated heparin, low-molecular-weight heparins, and vitamin K antagonists are drugs used routinely in the treatment and prophylaxis of thromboembolic disease. In addition to the well-known ADRs such as an increased bleeding tendency, these agents may cause CADR which necessitate institution of alternative therapies.
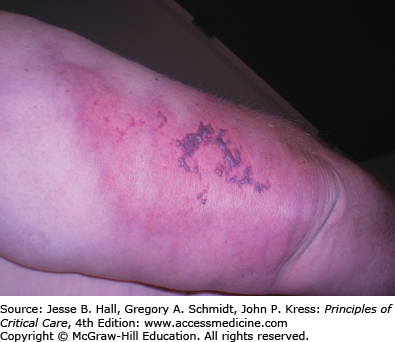
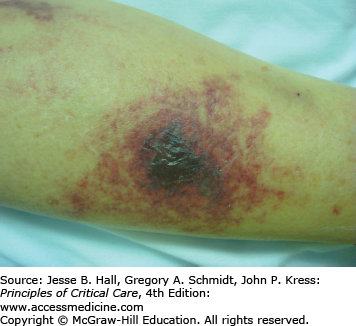
Heparin is a mucopolysaccharide that can induce various ADRs, including immediate reactions (eg, urticaria, asthma, and anaphylaxis), and delayed-type reactions (eg, erythematous plaques, skin necrosis, a generalized eruption, and thrombocytopenia).23 Delayed-type reactions manifest as erythematous, vesicular, or pruritic plaques at the injection site within 2 to 5 days of therapy, whereas heparin-induced skin necrosis (Fig. 129-7) develops between 5 and 10 days of therapy. Cutaneous reactions have also been described with the use of low-molecular-weight heparins and heparinoids.24 In patients with skin necrosis, heparin must be discontinued due to the increased risk of developing heparin-induced thrombocytopenia II.25 These patients usually have significant concurrent illnesses but no coagulation abnormalities. Histology shows fibrin deposition in venules and capillaries in the dermis and hypodermis, with necrosis. Intracutaneous testing is useful for the diagnosis of delayed reactions (erythematous plaques at heparin injection sites) but is contraindicated in the presence of skin necrosis. A positive skin test has been associated with heparin-induced IgG antibodies. There may be cross-reactivity between unfractionated heparin and low-molecular-weight heparins and heparinoids. Rates of 50% and 30%, respectively, have been reported.26 Therefore, treatment consists of discontinuing heparin and administering one of the newly developed direct thrombin inhibitors (argatroban and lepirudin) to provide protection during warfarin initiation.
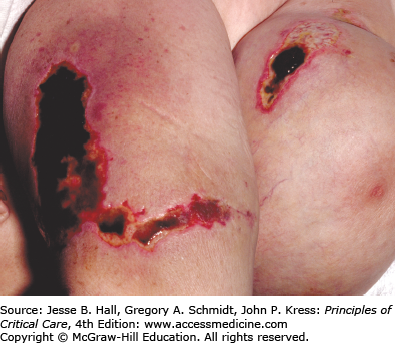
Warfarin inhibits the vitamin K–dependent clotting factors II, VII, IX, and X; it also inhibits the anticoagulant proteins C and S, thereby causing a transient hypercoagulable state.27 On rare occasions, skin necrosis (Fig. 129-8), which is potentially fatal (15% of cases), occurs with initiation of therapy before full anticoagulation is achieved. Three to ten days after starting therapy, pain precedes the appearance of ill-defined erythematous plaques, which progress into edematous, blue-black plaques with hemorrhagic bullae in the center. The lesions necrose, leaving central eschars. Necrosis commonly affects the breasts, abdomen, and thighs in females and the abdomen and thighs in males. Histopathology shows fibrin occluding dermal and hypodermal veins, with diffuse necrosis.28 This occurs more commonly in women. Other risk factors include a large loading dose, protein C deficiency, and the presence of the factor V Leiden mutation. Treatment consists of discontinuation of warfarin and administration of intravenous heparin. Some investigators have reported success with the use of protein C concentrate, prostacyclin, and factor VII.29 Lesions are treated as any other skin ulcer; however, when they are extensive, surgical debridement, grafting, or occasionally amputation may be necessary to prevent the development of infection and sepsis.
Herbal remedies are classified as dietary supplements. As such, they are exempt from the stringent evaluation and regulatory processes that drugs have to undergo to show efficacy, safety, and quality.30,31 It is not uncommon for patients admitted to the ICU to have been taking herbal medicines or supplements. Contrary to popular belief, herbal products may result in clinically significant adverse reactions and herbal-drug interactions. Some remedies have been found to contain traditional drugs, such as aspirin, paracetamol, mefenamic acid, diazepam, and triamcinolone, with corresponding adverse reactions. Contaminants, such as lead, arsenic, and other heavy metals, have been found in numerous herbal remedies and are a significant source of adverse reactions. Autoimmune thrombocytopenia has been reported in a patient receiving kelp tainted with arsenic.
Similarly, interactions with traditional drugs are a significant cause of concern in individuals taking herbal remedies concurrently with traditional medicines32 (estimated at 18% of the population). Components in herbal remedies such as St John’s wort and Kava-Kava, used for depression and anxiety, respectively, have been shown to inhibit monoamine oxidase activity. Interactions with drugs affecting the metabolism of neuroactive amines, such as the selective serotonin-reuptake inhibitors, are a potential problem. Therefore, coffee, tea, and foods containing tyramine (aged cheese, soy sauce, smoked meats, bananas, and avocados) should be avoided.
Because many of the mechanisms underlying acute hypersensitivity reactions are unclear, a careful history and evaluation is necessary. In addition, because the half-life of many of these components is not known, they should be discontinued immediately on admission to the hospital or the critical care unit.
Vasopressin, a nonselective vasoconstrictor, is used in the treatment of vasodilatory septic shock, hypotension unresponsive to catecholamines,33 and variceal hemorrhage.34 Uncommon adverse reactions to large-dose (0.2-0.4 U/min), centrally administered vasopressin include cardiac arrhythmias, ischemia, infarction, and bowel ischemia.46 When peripheral intravenous administration is used, accidental extravasation of the drug may result in skin necrosis (Fig. 129-9) and gangrene. This CADR may occur even at small doses (0.04 U/min) or when small quantities that are undetectable by the occlusion pressure monitor infiltrate the tissue. Administration through a central venous catheter is encouraged, even for small doses, to minimize the occurrence of this adverse reaction, although skin necrosis with low-dose vasopressin infusion through central venous route has been recently reported.36 Interestingly, skin necrosis does not occur with large-dose subcutaneous administration, as performed for diabetes insipidus. Mortality rate is usually high in patients with septic shock who develop necrotic skin lesions. Treatment consists of stopping the vasopressin infusion, surgically débriding the ischemic tissue, and applying wet dressings or grafting.
Diffuse ischemic skin lesions, defined as new areas of mottled or livid skin, have been found to occur in approximately 30% of patients during infusion of arginine-vasopressin, an alternative powerful vasopressor agent that is increasingly used in catecholamine-resistant vasodilatory shock. Sepsis and arterial occlusive disease are predisposing factors. These lesions, often seen on the limbs and trunk but also on the tongue, may lead to gangrene, necessitating debridement and amputation.37
Acute generalized exanthematous pustulosis (AGEP) is a rare, T-cell–mediated drug reaction that often presents within 1 to 5 days of starting the culprit medication. It is typically reported in association with antibacterial drugs (>90% of cases) including ampicillin, amoxicillin, quinolones, clindamycin, and sulfonamides. However, other topical and systemic medications, including corticosteroids, terbinafine, diltiazem, chloroquine, and herbal remedies, have also been implicated.
AGEP is clinically characterized by the acute onset of edematous erythema, followed by disseminated, small, nonfollicular sterile pustules which may coalesce into bullae (Fig. 129-10). Marked edema of the face, target-like lesions, blisters, and purpura may be seen, but are not typical of AGEP. Mild and nonerosive mucosal involvement occurs in 20% of patients. Patients have fever (T >38°C) and massive leukocytosis, sometimes with eosinophilia, but there is no internal organ involvement. Histologically, AGEP is characterized by a subcorneal or intraepidermal sterile pustule and marked spongiosis with a few necrotic keratinocytes.
AGEP is self-limiting and spontaneously resolves within 2 weeks. The 5% mortality rate, which is often reported, results from secondary infections in patients with other medical comorbidities. Treatment consists of discontinuation of the suspected drug and supportive therapy. Oral and topical steroids are not necessary; however, they may shorten the duration of the disease.
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS
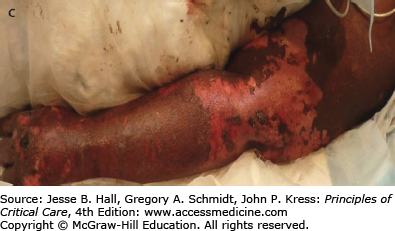
CADRs are classified by the WHO as severe when they are life threatening, require hospitalization, or lead to permanent disability. Stevens-Johnson syndrome (SJS) (Fig. 129-11) and toxic epidermal necrolysis (TEN) (Fig. 129-12) are rare, serious CADRs, with calculated incidences of 1 to 6 and 0.4 to 1.2 cases per million person-years, respectively.39 SJS and TEN are histopathologically identical diseases that are differentiated by the extent of epidermal detachment: less than 10% in SJS, 10% to 29% in transitional SJS/TEN, and greater than 30% in TEN. In addition to the hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms, SJS and TEN constitute the major groups of severe CADRs40 (Table 129-7).
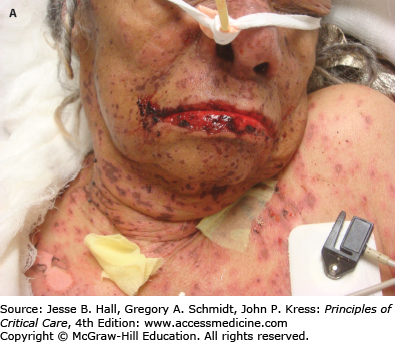
FIGURE 129-12
Toxic epidermal necrolysis (TEN). A. Mucosal involvement with erosions and hemorrhagic crusts. B. Large, intact bullae on erythematous skin. C. Detachment of full-thickness epidermis has led to areas of denuded skin. (Used with permission of Drs. Christopher R. Shea and Vesna Petronic-Rosic.)
Classification of Severe Cutaneous Adverse Drug Reactions
| SJS | TEN | Hypersensitivity/DRESS | |
|---|---|---|---|
| Mucous membrane | >90% | >90% | <30% |
| BSA involvementa | <10% | >30% | <10% |
| Erosions | Several sites | Several sites | Mouth and lips |
| Detachment of epidermis | Yes | Yes | No |
| Hyperkeratosis/desquamation | No | No | Usual |
| Neutropenia | No | 30% | No |
| Eosinophilia | No | No | 90% |
| Atypical lymphocytes | No | No | 30%-40% |
| Respiratory tract | Bronchial erosions/ARDS | Bronchial erosions/ARDS | Interstitial pneumonitis |
| Liver | Hepatitis 10% | Hepatitis 10% | Hepatitis 60% |
| Heart | No | No | Myocarditis |
| Lymph node enlarged | No | No | Usual |
SJS and TEN are caused almost exclusively by medications.41 The implicated medication is usually started within 3 weeks of the eruption. An increased risk for SJS and TEN has been found with the use of the following drugs: anticonvulsants (phenytoin, valproic acid, phenobarbital, and carbamazepine), antibiotics (sulfonamides and aminopenicillins), NSAIDs (oxicam derivatives), chlormezanone, allopurinol, and, interestingly, corticosteroids (Table 129-8).39 Drugs with long half-lives, known to be frequent culprits, have been associated with higher fatality rates.42 SJS is associated with a 5% mortality rate, whereas TEN, which is the most severe form of drug eruption known, has a mortality rate of approximately 30%.43 Mortality is mainly a result of respiratory complications and sepsis. Prognosis is influenced by age (>60 years), the presence of comorbidities, sepsis, and extent of body surface area involved.43-45 A prognostic scoring system, also known as SCORTEN, has been proposed (Table 129-9).46 TEN occurs more frequently in HIV-infected individuals, especially those receiving sulfonamides or nevirapine. Precipitation of SJS and TEN by infectious agents is much less common. Infection with Mycoplasma pneumoniae is the best documented.47
Factors Associated With SJS and TEN
| Drugs | Infections (Isolated Cases) |
|---|---|
| Antibiotics | Mycoplasma Pneumoniae |
| Histoplasmosis |
| Hepatitis A |
| Epstein-Barr virus |
| Coxsackievirus B5 |
| Anticonvulsants | Milkers’ nodules |
| Yersiniosis |
| Adenovirus |
| Gram-negative septicemia |
| |
| NSAIDs | |
| |
| |
| Allopurinol | |
| Chlormezanone | |
| Graft-versus-host disease |
SCORTEN: A Prognostic Scoring System for Patients With TEN
| Prognostic Factors | Points |
|---|---|
| Age >40 years | 1 |
| Heart rate >120 bpm | 1 |
| Cancer or hematologic malignancy | 1 |
| BSA involved on day 1 above 10% | 1 |
| Serum urea level (>10 mmol/L) | 1 |
| Serum bicarbonate level (<20 mmol/L) | 1 |
| Serum glucose level (>14 mmol/L) | 1 |
| SCORTEN | Mortality rate (%) |
| 0-1 | 3.2 |
| 2 | 12.1 |
| 3 | 35.8 |
| 4 | 58.3 |
| > or = 5 | 90 |
The pathogenesis of SJS and TEN remains unclear. Humoral and cell-mediated cytotoxicities, drug metabolites, and apoptotic mechanisms have been implicated, with inconsistent data. Experimental data suggest that activation of Fas (CD95) through its ligand, FasL (CD95L), results in caspase-mediated keratinocyte apoptosis, possibly an important pathophysiologic mechanism in TEN.48 Fas (CD95) is a cell surface receptor expressed on most cells, including keratinocytes, and can be activated by FasL, which is expressed by natural killer cells and activated T lymphocytes.49 FasL can also exist in a soluble form (sFasL), capable of inducing apoptosis.50 Soluble FasL results from metalloproteinase-mediated cleavage of cell surface FasL, and stimulation of peripheral blood mononuclear cells by an offending drug results in upregulated sFasL expression. Sera from SJS and TEN patients can induce Fas-FasL–mediated keratinocyte apoptosis in vitro, and contain elevated concentrations of sFasL when compared with control patients.51
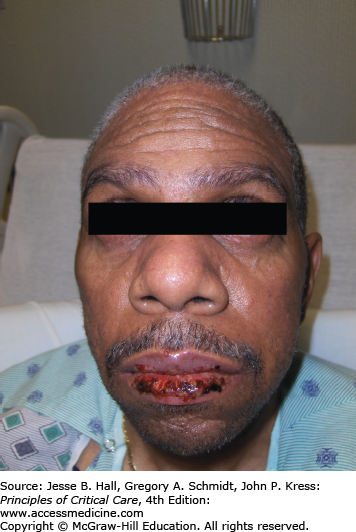
Initial symptoms of both SJS and TEN include pain, tenderness, or a burning sensation in the skin. These symptoms often begin abruptly and are associated with fever and general malaise. Over the next 1 to 3 days, ill-defined erythematous macules or diffuse erythema develop over the trunk and extremities. The palms and soles can be an early site of involvement. As the red areas enlarge, central dusky necrotic sites develop with subsequent bullae formation. Bullae result from edema occurring beneath the necrotic epidermis. As the disease progresses, sheets of full-thickness epidermis slough off, revealing dark red, moist dermis (resembling severe second-degree burns). The Nikolsky sign (separation of the skin with lateral traction) is positive and an important clinical diagnostic clue. Criteria to predict which patients with SJS are likely to progress to full-blown TEN are currently unavailable.
Mucous membrane involvement occurs in 85% to 95% of patients and may be the presenting sign.52 The oropharynx and conjunctivae are most affected, resulting in severe erosions and pain. Keratitis, ocular erosions, and symblepharon may result in blindness. Dysuria is a sign of urethral involvement. Erosions in the lower respiratory tract and the intestine have also been described.53 Involvement of the epithelium of the trachea, bronchi, or gastrointestinal tract increases morbidity. Laboratory, histopathology, and immunofluorescence findings are outlined in Table 129-10.
Clinical and Histopathological Features of SJS/TEN
| Laboratory Findings (% patients) | Histopathology | Immunofluorescence |
|---|---|---|
| Negative |
The clinical differential diagnosis includes staphylococcal scalded skin syndrome, acute GVHD, scarlet fever, erythema multiforme, and pemphigus vulgaris (Table 129-11). In all these cases, full-thickness epidermal necrosis is rare (except in GVHD, in which epidermal necrosis is accompanied by an abundant lymphocytic infiltrate).
Differential Diagnosis of SJS/TEN
| Differential Diagnosis | Cutaneous Findings | Histopathology/Immunofluorescence |
|---|---|---|
| Erythema multiforme | Asymptomatic, targetoid lesions | Interface dermatitis with individual apoptotic keratinocytes and a perivascular lymphocytic infiltrate/negative immunofluorescence |
| Staphylococcal scalded skin syndrome (SSSS) | Large areas of tender erythema, flaccid bullae followed by desquamation | Subcorneal blisters with no inflammatory cells/immunofluorescence negative |
| Acute generalized exanthematous pustulosis (AGEP) | Nonfollicular, sterile pustules within large areas of edematous erythema | Subcorneal or superficial epidermal blisters with neutrophils/immunofluorescence negative |
| Graft-versus-host disease | Generalized erythematous morbilliform eruption | Vacuolar interface dermatitis with satellite necrotic keratinocytes and epithelial atypia/negative immunofluorescence |
| Scarlet fever | Erythematous papules on the trunk with a “sandpaper-like” texture followed by desquamation of the hands and feet, strawberry tongue | Engorged capillaries and dilated lymphatic vessels, most prominent around hair follicles/negative immunofluorescence |
| Paraneoplastic pemphigus | Mucocutaneous involvement with tense and flaccid bullae intermixed with erosions. | Variable intraepidermal acantholysis, interface reaction with necrotic keratinocytes and vacuolar change, +/− subepidermal clefting/ IgG and C3 deposition between keratinocytes and at the dermoepidermal junction |
| Pemphigus vulgaris | Superficial flaccid bullae, erosions | Intraepidermal split/IgG and C3 deposition between keratinocytes |
| Bullous pemphigoid | Large tense bullae, urticarial wheals, serpiginous plaques | Subepidermal blister/linear deposition of IgG and C3 along basement membrane |
| Drug-induced linear IgA bullous dermatosis | Tense vesicles and bullae with annular configuration | Subepidermal blister with neutrophils/linear deposition of IgA along the dermoepidermal junction |
Erythema multiforme (Fig. 129-13) (previously referred to as erythema multiforme minor) is an acute, self-limited reaction characterized by asymptomatic, annular erythematous, or urticarial plaques with central areas of blistering and necrosis, resulting in the characteristic target lesions. Lesions usually develop on the extensor surfaces of the extremities and mucosae, and cover less than 10% of the body surface area. Outbreaks last for 1 to 4 weeks. Relapses are frequent. It is associated with recurrent herpes simplex virus infections in the great majority of cases and with M pneumoniae infection in a smaller subset.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree